Sac3 is an mRNA export factor that localizes to cytoplasmic fibrils of nuclear pore complex
- PMID: 12631707
- PMCID: PMC151563
- DOI: 10.1091/mbc.e02-08-0520
Sac3 is an mRNA export factor that localizes to cytoplasmic fibrils of nuclear pore complex
Abstract
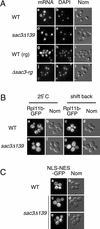
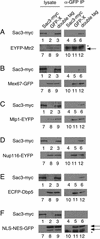
In eukaryotes, mRNAs are transcribed in the nucleus and exported to the cytoplasm for translation to occur. Messenger RNAs complexed with proteins referred to as ribonucleoparticles are recognized for nuclear export in part by association with Mex67, a key Saccharomyces cerevisiae mRNA export factor and homolog of human TAP/NXF1. Mex67, along with its cofactor Mtr2, is thought to promote ribonucleoparticle translocation by interacting directly with components of the nuclear pore complex (NPC). Herein, we show that the nuclear pore-associated protein Sac3 functions in mRNA export. Using a mutant allele of MTR2 as a starting point, we have identified a mutation in SAC3 in a screen for synthetic lethal interactors. Loss of function of SAC3 causes a strong nuclear accumulation of mRNA and synthetic lethality with a number of mRNA export mutants. Furthermore, Sac3 can be coimmunoprecipitated with Mex67, Mtr2, and other factors involved in mRNA export. Immunoelectron microscopy analysis shows that Sac3 localizes exclusively to cytoplasmic fibrils of the NPC. Finally, Mex67 accumulates at the nuclear rim when SAC3 is mutated, suggesting that Sac3 functions in Mex67 translocation through the NPC.
Figures



Similar articles
-
Role of Mex67-Mtr2 in the nuclear export of 40S pre-ribosomes.PLoS Genet. 2012;8(8):e1002915. doi: 10.1371/journal.pgen.1002915. Epub 2012 Aug 30. PLoS Genet. 2012. PMID: 22956913 Free PMC article.
-
A versatile interaction platform on the Mex67-Mtr2 receptor creates an overlap between mRNA and ribosome export.EMBO J. 2008 Jan 9;27(1):6-16. doi: 10.1038/sj.emboj.7601947. Epub 2007 Nov 29. EMBO J. 2008. PMID: 18046452 Free PMC article.
-
Nuclear export of ribosomal 60S subunits by the general mRNA export receptor Mex67-Mtr2.Mol Cell. 2007 Apr 13;26(1):51-62. doi: 10.1016/j.molcel.2007.02.018. Mol Cell. 2007. PMID: 17434126
-
Nuclear export of mRNA.FEBS Lett. 2001 Jun 8;498(2-3):150-6. doi: 10.1016/s0014-5793(01)02482-6. FEBS Lett. 2001. PMID: 11412847 Review.
-
A novel family of nuclear transport receptors mediates the export of messenger RNA to the cytoplasm.Eur J Cell Biol. 2002 Nov;81(11):577-84. doi: 10.1078/0171-9335-00273. Eur J Cell Biol. 2002. PMID: 12498157 Review.
Cited by
-
A genetic screen in Saccharomyces cerevisiae identifies new genes that interact with mex67-5, a temperature-sensitive allele of the gene encoding the mRNA export receptor.Mol Genet Genomics. 2009 Jan;281(1):125-34. doi: 10.1007/s00438-008-0402-x. Epub 2008 Nov 26. Mol Genet Genomics. 2009. PMID: 19034519
-
Evidence that the Arabidopsis Ubiquitin C-terminal Hydrolases 1 and 2 associate with the 26S proteasome and the TREX-2 complex.Plant Signal Behav. 2012 Nov;7(11):1415-9. doi: 10.4161/psb.21899. Epub 2012 Sep 5. Plant Signal Behav. 2012. PMID: 22951400 Free PMC article.
-
THSC/TREX-2 deficiency causes replication stress and genome instability in Caenorhabditis elegans.J Cell Sci. 2021 Oct 15;134(20):jcs258435. doi: 10.1242/jcs.258435. Epub 2021 Oct 22. J Cell Sci. 2021. PMID: 34553761 Free PMC article.
-
A genetic interaction map of RNA-processing factors reveals links between Sem1/Dss1-containing complexes and mRNA export and splicing.Mol Cell. 2008 Dec 5;32(5):735-46. doi: 10.1016/j.molcel.2008.11.012. Mol Cell. 2008. PMID: 19061648 Free PMC article.
-
Mediator and TREX-2: Emerging links between transcription initiation and mRNA export.Nucleus. 2016 Apr 25;7(2):126-31. doi: 10.1080/19491034.2016.1169352. Epub 2016 Mar 30. Nucleus. 2016. PMID: 27028218 Free PMC article.
References
-
- Baβler J, Grandi P, Gadal O, Lessmann T, Petfalski E, Tollervey D, Lechner J, Hurt E. Identification of a 60S preribosomal particle that is closely linked to nuclear export. Mol Cell. 2001;8:517–529. - PubMed
-
- Bauer A, Kölling R. Characterization of the SAC3 gene of Saccharomyces cerevisiae. Yeast. 1996a;12:965–975. - PubMed
-
- Bauer A, Kölling R. The SAC3 gene encodes a nuclear protein required for normal progression of mitosis. J Cell Sci. 1996b;109:1575–1583. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

