Isoprenylcysteine carboxyl methyltransferase activity modulates endothelial cell apoptosis
- PMID: 12631708
- PMCID: PMC151564
- DOI: 10.1091/mbc.e02-07-0390
Isoprenylcysteine carboxyl methyltransferase activity modulates endothelial cell apoptosis
Abstract
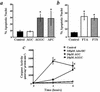
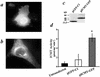
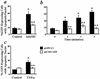
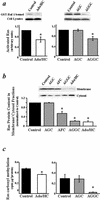
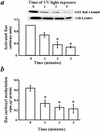
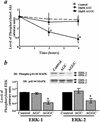
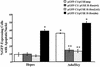
Extracellular ATP, adenosine (Ado), and adenosine plus homocysteine (Ado/HC) cause apoptosis of cultured pulmonary artery endothelial cells through the enhanced formation of intracellular S-adenosylhomocysteine and disruption of focal adhesion complexes. Because an increased intracellular ratio of S-adenosylhomocysteine/S-adenosylmethionine favors inhibition of methylation, we hypothesized that Ado/HC might act by inhibition of isoprenylcysteine-O-carboxyl methyltransferase (ICMT). We found that N-acetyl-S-geranylgeranyl-L-cysteine (AGGC) and N-acetyl-S-farnesyl-L-cysteine (AFC), which inhibit ICMT by competing with endogenous substrates for methylation, caused apoptosis. Transient overexpression of ICMT inhibited apoptosis caused by Ado/HC, UV light exposure, or tumor necrosis factor-alpha. Because the small GTPase, Ras, is a substrate for ICMT and may modulate apoptosis, we also hypothesized that inhibition of ICMT with Ado/HC or AGGC might cause endothelial apoptosis by altering Ras activation. We found that ICMT inhibition decreased Ras methylation and activity and the activation of the downstream signaling molecules Akt, ERK-1, and ERK-2. Furthermore, overexpression of wild-type or dominant active H-Ras blocked Ado/HC-induced apoptosis. These findings suggest that inhibition of ICMT causes endothelial cell apoptosis by attenuation of Ras GTPase methylation and activation and its downstream antiapoptotic signaling pathway.
Figures

Similar articles
-
Isoprenylcysteine carboxyl methyltransferase modulates endothelial monolayer permeability: involvement of RhoA carboxyl methylation.Circ Res. 2004 Feb 20;94(3):306-15. doi: 10.1161/01.RES.0000113923.85084.C1. Epub 2003 Dec 29. Circ Res. 2004. PMID: 14699010
-
Inhibition of ICMT induces endothelial cell apoptosis through GRP94.Am J Respir Cell Mol Biol. 2007 Jul;37(1):20-30. doi: 10.1165/rcmb.2006-0301SM. Epub 2007 Mar 8. Am J Respir Cell Mol Biol. 2007. PMID: 17347446 Free PMC article.
-
Role of isoprenylcysteine carboxyl methyltransferase in tumor necrosis factor-alpha stimulation of expression of vascular cell adhesion molecule-1 in endothelial cells.Arterioscler Thromb Vasc Biol. 2002 May 1;22(5):759-64. doi: 10.1161/01.atv.0000015884.61894.dc. Arterioscler Thromb Vasc Biol. 2002. PMID: 12006387
-
Pleiotropic effects of methionine adenosyltransferases deregulation as determinants of liver cancer progression and prognosis.J Hepatol. 2013 Oct;59(4):830-41. doi: 10.1016/j.jhep.2013.04.031. Epub 2013 May 7. J Hepatol. 2013. PMID: 23665184 Review.
-
Isoprenyl carboxyl methyltransferase inhibitors: a brief review including recent patents.Amino Acids. 2017 Sep;49(9):1469-1485. doi: 10.1007/s00726-017-2454-x. Epub 2017 Jun 19. Amino Acids. 2017. PMID: 28631011 Free PMC article. Review.
Cited by
-
Involvement and mechanism of DGAT2 upregulation in the pathogenesis of alcoholic fatty liver disease.J Lipid Res. 2010 Nov;51(11):3158-65. doi: 10.1194/jlr.M007948. Epub 2010 Aug 25. J Lipid Res. 2010. PMID: 20739640 Free PMC article.
-
Folate-regulated changes in gene expression in the anterior neural tube of folate binding protein-1 (Folbp1)-deficient murine embryos.Neurochem Res. 2004 Jun;29(6):1105-12. doi: 10.1023/b:nere.0000023597.37698.13. Neurochem Res. 2004. PMID: 15176467
-
Release of soluble E-selectin from activated endothelial cells upon apoptosis.Lung. 2006 Sep-Oct;184(5):259-66. doi: 10.1007/s00408-005-2589-5. Lung. 2006. PMID: 17235725
-
Clostridium difficile toxin B differentially affects GPCR-stimulated Ca2+ responses in macrophages: independent roles for Rho and PLA2.J Leukoc Biol. 2010 Jun;87(6):1041-57. doi: 10.1189/jlb.1108708. Epub 2010 Mar 3. J Leukoc Biol. 2010. PMID: 20200401 Free PMC article.
-
RAS-converting enzyme 1-mediated endoproteolysis is required for trafficking of rod phosphodiesterase 6 to photoreceptor outer segments.Proc Natl Acad Sci U S A. 2011 May 24;108(21):8862-6. doi: 10.1073/pnas.1103627108. Epub 2011 May 9. Proc Natl Acad Sci U S A. 2011. PMID: 21555557 Free PMC article.
References
-
- Bellas RE, Harrington EO, Sheahan KL, Newton J, Marcus C, Rounds S. FAK blunts adenosine-homocysteine-induced endothelial cell apoptosis: requirement for PI 3-kinase. Am J Physiol. 2002;282:L1135–L1142. - PubMed
-
- Bergo MO, Leung GK, Ambroziak P, Otto JC, Casey PJ, Gomes AQ, Seabra MC, Young SG. Isoprenylcysteine carboxyl methyltransferase deficiency in mice. J Biol Chem. 2001;276:5841–5845. - PubMed
-
- Bergo MO, Leung GK, Ambroziak P, Otto JC, Casey PJ, Young SG. Targeted inactivation of the isoprenylcysteine carboxyl methyltransferase gene causes mislocalization of K-Ras in mammalian cells. J Biol Chem. 2000;275:17605–17610. - PubMed
-
- Boivin D, Bilodeau D, Beliveau R. Regulation of cytoskeletal functions by Rho small GTP-binding proteins in normal and cancer cells. Can J Pharmacol. 1996;74:801–810. - PubMed
-
- Chatterjee M, Wu S. Cell line dependent involvement of ceramide in ultraviolet light-induced apoptosis. Mol Cell Biochem. 2001;219:21–27. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

