HIV-1 envelope proteins complete their folding into six-helix bundles immediately after fusion pore formation
- PMID: 12631714
- PMCID: PMC151570
- DOI: 10.1091/mbc.e02-09-0573
HIV-1 envelope proteins complete their folding into six-helix bundles immediately after fusion pore formation
Abstract
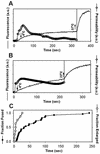
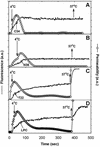
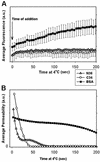
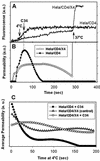
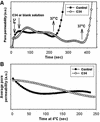
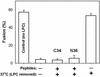
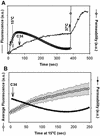
Fusion proteins of many viruses, including HIV-1 envelope protein (Env), fold into six-helix bundle structures. Fusion between individual Env-expressing cells and target cells was studied by fluorescence microscopy, and a temperature jump technique, to determine whether folding of Env into a bundle is complete by the time fusion pores have formed. Lowering temperature to 4 degrees C immediately after a pore opened halted pore growth, which quickly resumed when temperature was raised again. HIV gp41-derived peptides that inhibit bundle formation (C34 or N36) caused the cold-arrested pore to quickly and irreversibly close, demonstrating that bundle formation is not complete by the time a pore has formed. In contrast, lowering the temperature to an intermediate value also halted pore growth, but the pore was not closed by the bundle-inhibiting peptides, and it enlarged when temperature was again elevated. This latter result shows that bundle formation is definitely required for the fusion process, but surprisingly, some (if not all) bundle formation occurs after a pore has formed. It is concluded that an essential function of the bundle is to stabilize the pore against collapse and ensure its growth.
Figures


Similar articles
-
HIV-1 gp41 six-helix bundle formation occurs rapidly after the engagement of gp120 by CXCR4 in the HIV-1 Env-mediated fusion process.Biochemistry. 2001 Oct 16;40(41):12231-6. doi: 10.1021/bi0155596. Biochemistry. 2001. PMID: 11591141
-
Human immunodeficiency virus type 1 Env with an intersubunit disulfide bond engages coreceptors but requires bond reduction after engagement to induce fusion.J Virol. 2003 May;77(10):5829-36. doi: 10.1128/jvi.77.10.5829-5836.2003. J Virol. 2003. PMID: 12719576 Free PMC article.
-
The cytoplasmic tail slows the folding of human immunodeficiency virus type 1 Env from a late prebundle configuration into the six-helix bundle.J Virol. 2005 Jan;79(1):106-15. doi: 10.1128/JVI.79.1.106-115.2005. J Virol. 2005. PMID: 15596806 Free PMC article.
-
The stability of the intact envelope glycoproteins is a major determinant of sensitivity of HIV/SIV to peptidic fusion inhibitors.J Mol Biol. 2004 Jun 25;340(1):9-14. doi: 10.1016/j.jmb.2004.04.027. J Mol Biol. 2004. PMID: 15184018
-
The mechanism of inhibition of HIV-1 env-mediated cell-cell fusion by recombinant cores of gp41 ectodomain.Virology. 2002 Oct 10;302(1):174-84. doi: 10.1006/viro.2002.1593. Virology. 2002. PMID: 12429526
Cited by
-
Different infectivity of HIV-1 strains is linked to number of envelope trimers required for entry.PLoS Pathog. 2015 Jan 8;11(1):e1004595. doi: 10.1371/journal.ppat.1004595. eCollection 2015 Jan. PLoS Pathog. 2015. PMID: 25569556 Free PMC article.
-
Kinetic analyses of the surface-transmembrane disulfide bond isomerization-controlled fusion activation pathway in Moloney murine leukemia virus.J Virol. 2005 Nov;79(22):13856-64. doi: 10.1128/JVI.79.22.13856-13864.2005. J Virol. 2005. PMID: 16254321 Free PMC article.
-
Crystal structure of HIV-1 gp41 including both fusion peptide and membrane proximal external regions.PLoS Pathog. 2010 May 6;6(5):e1000880. doi: 10.1371/journal.ppat.1000880. PLoS Pathog. 2010. PMID: 20463810 Free PMC article.
-
The Stabilities of the Soluble Ectodomain and Fusion Peptide Hairpins of the Influenza Virus Hemagglutinin Subunit II Protein Are Positively Correlated with Membrane Fusion.Biochemistry. 2018 Sep 18;57(37):5480-5493. doi: 10.1021/acs.biochem.8b00764. Epub 2018 Sep 5. Biochemistry. 2018. PMID: 30141905 Free PMC article.
-
Receptor-induced thiolate couples Env activation to retrovirus fusion and infection.PLoS Pathog. 2007 Dec 21;3(12):e198. doi: 10.1371/journal.ppat.0030198. PLoS Pathog. 2007. PMID: 18260686 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

