Specification of actin filament function and molecular composition by tropomyosin isoforms
- PMID: 12631719
- PMCID: PMC151575
- DOI: 10.1091/mbc.e02-04-0244
Specification of actin filament function and molecular composition by tropomyosin isoforms
Abstract
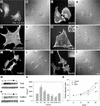
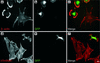
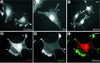
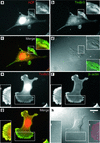
The specific functions of greater than 40 vertebrate nonmuscle tropomyosins (Tms) are poorly understood. In this article we have tested the ability of two Tm isoforms, TmBr3 and the human homologue of Tm5 (hTM5(NM1)), to regulate actin filament function. We found that these Tms can differentially alter actin filament organization, cell size, and shape. hTm5(NM1) was able to recruit myosin II into stress fibers, which resulted in decreased lamellipodia and cellular migration. In contrast, TmBr3 transfection induced lamellipodial formation, increased cellular migration, and reduced stress fibers. Based on coimmunoprecipitation and colocalization studies, TmBr3 appeared to be associated with actin-depolymerizing factor/cofilin (ADF)-bound actin filaments. Additionally, the Tms can specifically regulate the incorporation of other Tms into actin filaments, suggesting that selective dimerization may also be involved in the control of actin filament organization. We conclude that Tm isoforms can be used to specify the functional properties and molecular composition of actin filaments and that spatial segregation of isoforms may lead to localized specialization of actin filament function.
Figures






References
-
- Amano M, Ito M, Kimura K, Fukata Y, Chihara K, Nakano T, Matsuura Y, Kaibuchi K. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase) J Biol Chem. 1996;271:20246–20249. - PubMed
-
- Arber S, Barbayannis FA, Hanser H, Schneider C, Stanyon CA, Bernard O, Caroni P. Regulation of actin dynamics through phosphorylation of cofilin by LIM-kinase. Nature. 1998;393:805–809. - PubMed
-
- Bamburg JR. Proteins of the ADF/cofilin family: essential regulators of actin dynamics. Annu Rev Cell Dev Biol. 1999;15:185–230. - PubMed
-
- Beisel KW, Kennedy JE. Identification of novel alternatively spliced isoforms of the tropomyosin-encoding gene, TMnm, in the rat cochlea. Gene. 1994;143:251–256. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

