Loss of caveolin-1 gene expression accelerates the development of dysplastic mammary lesions in tumor-prone transgenic mice
- PMID: 12631721
- PMCID: PMC151577
- DOI: 10.1091/mbc.e02-08-0503
Loss of caveolin-1 gene expression accelerates the development of dysplastic mammary lesions in tumor-prone transgenic mice
Abstract
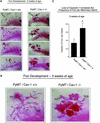
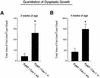
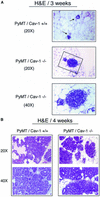
Caveolin-1 is the principal structural component of caveolae microdomains, which represent a subcompartment of the plasma membrane. Several independent lines of evidence support the notion that caveolin-1 functions as a suppressor of cell transformation. For example, the human CAV-1 gene maps to a suspected tumor suppressor locus (D7S522/7q31.1) that is frequently deleted in a number of carcinomas, including breast cancers. In addition, up to 16% of human breast cancers harbor a dominant-negative mutation, P132L, in the CAV-1 gene. Despite these genetic associations, the tumor suppressor role of caveolin-1 still remains controversial. To directly assess the in vivo transformation suppressor activity of the caveolin-1 gene, we interbred Cav-1 (-/-) null mice with tumor-prone transgenic mice (MMTV-PyMT) that normally develop multifocal dysplastic lesions throughout the entire mammary tree. Herein, we show that loss of caveolin-1 gene expression dramatically accelerates the development of these multifocal dysplastic mammary lesions. At 3 wk of age, loss of caveolin-1 resulted in an approximately twofold increase in the number of lesions (foci per gland; 3.3 +/- 1.0 vs. 7.0 +/- 1.2) and an approximately five- to sixfold increase in the total area occupied by these lesions. Similar results were obtained at 4 wk of age. However, complete loss of caveolin-1 was required to accelerate the appearance of these dysplastic mammary lesions, because Cav-1 (+/-) heterozygous mice did not show any increases in foci development. We also show that loss of caveolin-1 increases the extent and the histological grade of these mammary lesions and facilitates the development of papillary projections in the mammary ducts. Finally, we demonstrate that cyclin D1 expression levels are dramatically elevated in Cav-1 (-/-) null mammary lesions, consistent with the accelerated appearance and growth of these dysplastic foci. This is the first in vivo demonstration that caveolin-1 can function as a transformation suppressor gene.
Figures






Similar articles
-
Caveolin-1 mutations (P132L and null) and the pathogenesis of breast cancer: caveolin-1 (P132L) behaves in a dominant-negative manner and caveolin-1 (-/-) null mice show mammary epithelial cell hyperplasia.Am J Pathol. 2002 Oct;161(4):1357-69. doi: 10.1016/S0002-9440(10)64412-4. Am J Pathol. 2002. PMID: 12368209 Free PMC article.
-
Caveolin-1 gene disruption promotes mammary tumorigenesis and dramatically enhances lung metastasis in vivo. Role of Cav-1 in cell invasiveness and matrix metalloproteinase (MMP-2/9) secretion.J Biol Chem. 2004 Dec 3;279(49):51630-46. doi: 10.1074/jbc.M409214200. Epub 2004 Sep 7. J Biol Chem. 2004. PMID: 15355971
-
Absence of caveolin-1 sensitizes mouse skin to carcinogen-induced epidermal hyperplasia and tumor formation.Am J Pathol. 2003 Jun;162(6):2029-39. doi: 10.1016/S0002-9440(10)64335-0. Am J Pathol. 2003. PMID: 12759258 Free PMC article.
-
Caveolin-1 in oncogenic transformation, cancer, and metastasis.Am J Physiol Cell Physiol. 2005 Mar;288(3):C494-506. doi: 10.1152/ajpcell.00458.2004. Am J Physiol Cell Physiol. 2005. PMID: 15692148 Review.
-
Caveolin-1 in breast cancer.Cancer Biol Ther. 2004 Oct;3(10):931-41. doi: 10.4161/cbt.3.10.1147. Epub 2004 Oct 28. Cancer Biol Ther. 2004. PMID: 15539932 Review.
Cited by
-
Caveolin-1 is Associated with Tumor Progression and Confers a Multi-Modality Resistance Phenotype in Pancreatic Cancer.Sci Rep. 2015 Jun 12;5:10867. doi: 10.1038/srep10867. Sci Rep. 2015. PMID: 26065715 Free PMC article.
-
Expression of caveolin 1 in oral squamous cell carcinoma.J Oral Maxillofac Pathol. 2024 Apr-Jun;28(2):200-204. doi: 10.4103/jomfp.jomfp_310_23. Epub 2024 Jul 11. J Oral Maxillofac Pathol. 2024. PMID: 39157842 Free PMC article.
-
MMTV promoter-regulated caveolin-1 overexpression yields defective parenchymal epithelia in multiple exocrine organs of transgenic mice.Exp Mol Pathol. 2010 Aug;89(1):9-19. doi: 10.1016/j.yexmp.2010.03.009. Epub 2010 Apr 22. Exp Mol Pathol. 2010. PMID: 20399205 Free PMC article.
-
Caveola-forming proteins caveolin-1 and PTRF in prostate cancer.Nat Rev Urol. 2013 Sep;10(9):529-36. doi: 10.1038/nrurol.2013.168. Epub 2013 Aug 13. Nat Rev Urol. 2013. PMID: 23938946 Review.
-
Phosphocaveolin-1 enforces tumor growth and chemoresistance in rhabdomyosarcoma.PLoS One. 2014 Jan 10;9(1):e84618. doi: 10.1371/journal.pone.0084618. eCollection 2014. PLoS One. 2014. PMID: 24427291 Free PMC article.
References
-
- Baribault H, Wilson-Heiner M, Muller W, Penner J, Bakhiet N. Functional analysis of mouse keratin 8 in polyoma middle T-induced mammary gland tumors. Transgenic Res. 1997;6:359–367. - PubMed
-
- Bartkova J, Lukas J, Muller H, Lutzhoft D, Strauss M, Bartek J. Cyclin D1 protein expression and function in human breast cancer. Int J Cancer. 1994;57:353–361. - PubMed
-
- Bugge TH, Lund LR, Kombrinck KK, Nielsen BS, Holmback K, Drew AF, Flick MJ, Witte DP, Dano K, Degen JL. Reduced metastasis of Polyoma virus middle T antigen-induced mammary cancer in plasminogen-deficient mice. Oncogene. 1998;16:3097–3104. - PubMed
-
- Cardiff RD, et al. The mammary pathology of genetically engineered mice: the consensus report and recommendations from the Annapolis meeting. Oncogene. 2000;19:968–988. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

