Dynamin participates in focal extracellular matrix degradation by invasive cells
- PMID: 12631724
- PMCID: PMC151580
- DOI: 10.1091/mbc.e02-05-0308
Dynamin participates in focal extracellular matrix degradation by invasive cells
Abstract
The degradation of extracellular matrix (ECM) by matrix metalloproteases is crucial in physiological and pathological cell invasion alike. Degradation occurs at specific sites where invasive cells make contact with the ECM via specialized plasma membrane protrusions termed invadopodia. Herein, we show that the dynamin 2 (Dyn2), a GTPase implicated in the control of actin-driven cytoskeletal remodeling events and membrane transport, is necessary for focalized matrix degradation at invadopodia. Dynamin was inhibited by using two approaches: 1) expression of dominant negative GTPase-impaired or proline-rich domain-deleted Dyn2 mutants; and 2) inhibition of the dynamin regulator calcineurin by cyclosporin A. In both cases, the number and extension of ECM degradation foci were drastically reduced. To understand the site and mechanism of dynamin action, the cellular structures devoted to ECM degradation were analyzed by correlative confocal light-electron microscopy. Invadopodia were found to be organized into a previously undescribed ECM-degradation structure consisting of a large invagination of the ventral plasma membrane surface in close spatial relationship with the Golgi complex. Dyn2 seemed to be concentrated at invadopodia.
Figures


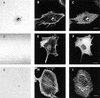
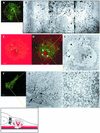

References
-
- Artalejo CR, Elhamdani A, Palfrey HC. Calmodulin is the divalent cation receptor for rapid endocytosis, but not exocytosis, in adrenal chromaffin cells. Neuron. 1996;16:195–205. - PubMed
-
- Awumey EM, Moonga BS, Sodam BR, Koval AP, Adebanjo OA, Kumegawa M, Zaidi M, Epstein S. Molecular and functional evidence for calcineurin-A α and β isoforms in the osteoclast: novel insights into cyclosporin A action on bone resorption. Biochem Biophys Res Commun. 1999;254:248–252. - PubMed
-
- Ayala I, Babia T, Baldassarre M, Pompeo A, Fabra A, Kok JW, Luini A, Buccione R, Egea G. Morphological and biochemical analysis of the secretory pathway in melanoma cells with distinct metastatic potential. FEBS Lett. 1999;451:315–320. - PubMed
-
- Backer JM. Phosphoinositide 3-kinases and the regulation of vesicular trafficking. Mol Cell Biol Res Commun. 2000;3:193–204. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

