Essential roles for GPI-anchored proteins in African trypanosomes revealed using mutants deficient in GPI8
- PMID: 12631733
- PMCID: PMC151589
- DOI: 10.1091/mbc.e02-03-0167
Essential roles for GPI-anchored proteins in African trypanosomes revealed using mutants deficient in GPI8
Abstract
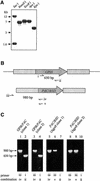
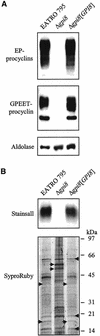
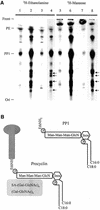
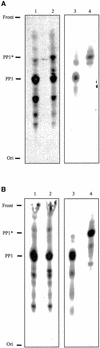
The survival of Trypanosoma brucei, the causative agent of Sleeping Sickness and Nagana, is facilitated by the expression of a dense surface coat of glycosylphosphatidylinositol (GPI)-anchored proteins in both its mammalian and tsetse fly hosts. We have characterized T. brucei GPI8, the gene encoding the catalytic subunit of the GPI:protein transamidase complex that adds preformed GPI anchors onto nascent polypeptides. Deletion of GPI8 (to give Deltagpi8) resulted in the absence of GPI-anchored proteins from the cell surface of procyclic form trypanosomes and accumulation of a pool of non-protein-linked GPI molecules, some of which are surface located. Procyclic Deltagpi8, while viable in culture, were unable to establish infections in the tsetse midgut, confirming that GPI-anchored proteins are essential for insect-parasite interactions. Applying specific inducible GPI8 RNAi with bloodstream form parasites resulted in accumulation of unanchored variant surface glycoprotein and cell death with a defined multinuclear, multikinetoplast, and multiflagellar phenotype indicative of a block in cytokinesis. These data show that GPI-anchored proteins are essential for the viability of bloodstream form trypanosomes even in the absence of immune challenge and imply that GPI8 is important for proper cell cycle progression.
Figures



References
-
- Acosta-Serrano A, Cole RN, Englund PT. Killing of Trypanosoma brucei by concanavalin A: structural basis of resistance in glycosylation mutants. J Mol Biol. 2000;304:633–644. - PubMed
-
- Barrett AJ, Rawlings ND. Evolutionary lines of cysteine peptidases. Biol Chem. 2001;382:727–733. - PubMed
-
- Baumann NA, Vidugiriene J, Machamer CE, Menon AK. Cell surface display and intracellular trafficking of free glycosylphosphatidylinositols in mammalian cells. J Biol Chem. 2000;275:7378–7389. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

