Quantitative analysis of herpes simplex virus reactivation in vivo demonstrates that reactivation in the nervous system is not inhibited at early times postinoculation
- PMID: 12634371
- PMCID: PMC150616
- DOI: 10.1128/jvi.77.7.4127-4138.2003
Quantitative analysis of herpes simplex virus reactivation in vivo demonstrates that reactivation in the nervous system is not inhibited at early times postinoculation
Abstract
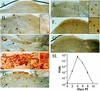
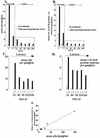
Recent studies utilizing an ex vivo mouse model of herpes simplex virus (HSV) reactivation have led to the hypothesis that, under physiologic conditions inducing viral reactivation, the immune cells within the infected ganglion block the viral replication cycle and maintain the viral genome in a latent state. One prediction from the ex vivo study is that reactivation in ganglia in vivo would be inhibited at early times postinoculation, when the numbers of inflammatory cells in the ganglia are greatest. To distinguish between an effect of the immune infiltrates on (i) infectious virus produced and/or recovered in the ganglion and (ii) the number of neurons undergoing lytic transcriptional activity (reactivating), an assay to quantify the number of neurons expressing lytic viral protein in ganglia in vivo was developed. Infectious virus and HSV protein-positive neurons were quantified from days 9 through 240 postinoculation in latently infected trigeminal ganglia before and at 22 h after hyperthermic-stress-induced reactivation. Significant increases in the amount of virus and the number of positive neurons were detected poststress in ganglia at all times examined. Unexpectedly, the greatest levels of reactivation occurred at the times examined most proximal to inoculation. Acyclovir was utilized to stop residual acute-phase virus production, and this treatment did not reduce the level of reactivation on day 14. Thus, the virus measured after induction was a product of reactivation. These data indicate that, in contrast to observations in the ex vivo model, immune cells in the ganglia during the resolution of acute infection do not inhibit reactivation of the virus in ganglia in vivo.
Figures







References
-
- Bauer, D., and R. Tampe. 2002. Herpes viral proteins blocking the transporter associated with antigen processing TAP: from genes to function and structure. Curr. Top. Microbiol. Immunol. 269:87-99. - PubMed
-
- Blyth, W. A., T. J. Hill, H. J. Field, and D. A. Harbour. 1976. Reactivation of herpes simplex virus infection by ultraviolet light and possible involvement of prostaglandins. J. Gen. Virol. 33:547-550. - PubMed
-
- Dent, J. A., A. G. Polson, and M. W. Klymkowsky. 1989. A whole-mount immunocytochemical analysis of the expression of the intermediate filament protein vimentin in Xenopus. Development 105:61-74. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

