Role of CD8(+) lymphocytes in control and clearance of measles virus infection of rhesus monkeys
- PMID: 12634396
- PMCID: PMC150640
- DOI: 10.1128/jvi.77.7.4396-4400.2003
Role of CD8(+) lymphocytes in control and clearance of measles virus infection of rhesus monkeys
Abstract
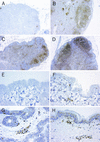
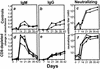
The creation of an improved vaccine for global measles control will require an understanding of the immune mechanisms of measles virus containment. To assess the role of CD8(+) cytotoxic T lymphocytes in measles virus clearance, rhesus monkeys were depleted of CD8(+) lymphocytes by monoclonal anti-CD8 antibody infusion and challenged with wild-type measles virus. The CD8(+) lymphocyte-depleted animals exhibited a more extensive rash, higher viral loads at the peak of virus replication, and a longer duration of viremia than did the control antibody-treated animals. These findings indicate a central role for CD8(+) lymphocytes in the control of measles virus infections and the importance of eliciting a cell-mediated immune response in new measles vaccine strategies.
Figures



References
-
- Anonymous. 1999. Global measles control and regional elimination, 1998-1999. Morb. Mortal. Wkly. Rep. 48:1124-1130. - PubMed
-
- Auwaerter, P. G., P. A. Rota, W. R. Elkins, R. J. Adams, T. DeLozier, Y. Shi, W. J. Bellini, B. R. Murphy, and D. E. Griffin. 1999. Measles virus infection in rhesus macaques: altered immune responses and comparison of the virulence of six different virus strains. J. Infect. Dis. 180:950-958. - PubMed
-
- Burnet, F. M. 1968. Measles as an index of immunological function. Lancet ii:610-613. - PubMed
-
- Chen, R. T., L. E. Markowitz, P. Albrecht, J. A. Stewart, L. M. Mofenson, S. R. Preblud, and W. A. Orenstein. 1990. Measles antibody: reevaluation of protective titers. J. Infect. Dis. 162:1036-1042. - PubMed
-
- Forthal, D. N., G. Landucci, A. Habis, M. Zartarian, J. Katz, and J. G. Tilles. 1994. Measles virus-specific functional antibody responses and viremia during acute measles. J. Infect. Dis. 169:1377-1380. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

