Disassembly of human immunodeficiency virus type 1 cores in vitro reveals association of Nef with the subviral ribonucleoprotein complex
- PMID: 12634398
- PMCID: PMC150647
- DOI: 10.1128/jvi.77.7.4409-4414.2003
Disassembly of human immunodeficiency virus type 1 cores in vitro reveals association of Nef with the subviral ribonucleoprotein complex
Erratum in
- J Virol. 2004 Apr;78(8):4384
Abstract
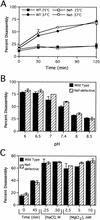
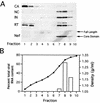
The human immunodeficiency virus type 1 (HIV-1) virulence factor Nef enhances viral infectivity in single-cycle infection assays and accelerates HIV-1 replication in vitro. It has been reported that the effects of Nef are mediated early after viral entry and before the completion of reverse transcription, as viral DNA synthesis is strongly attenuated during infection by Nef-defective virions. Our previous work has demonstrated that Nef is associated with mature HIV-1 cores, implicating Nef in the regulation of HIV-1 core stability. Here we report a comparative analysis of HIV-1 cores isolated from wild-type and Nef-defective particles. We observed no effect of Nef on HIV-1 core structure or stability; however, Nef cosedimented with a subviral ribonucleoprotein complex following dissociation of CA. These results indicate that Nef interacts tightly with an internal component of the HIV-1 core. They further suggest that virion-associated Nef may facilitate an early step in HIV-1 infection following dissociation of the viral capsid in the target cell.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

