Dynamics of the interaction between the insulin receptor and protein tyrosine-phosphatase 1B in living cells
- PMID: 12634852
- PMCID: PMC1315895
- DOI: 10.1038/sj.embor.embor767
Dynamics of the interaction between the insulin receptor and protein tyrosine-phosphatase 1B in living cells
Abstract
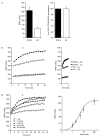
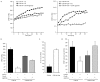
The dynamics of the interaction of the insulin receptor with a substrate-trapping mutant of protein-tyrosine phosphatase 1B (PTP1B) were monitored in living human embryonic kidney cells using bioluminescence resonance energy transfer (BRET). Insulin dose-dependently stimulates this interaction, which could be followed in real time for more than 30 minutes. The effect of insulin on the BRET signal could be detected at early time-points (30 seconds), suggesting that in intact cells the tyrosine-kinase activity of the insulin receptor is tightly controlled by PTP1B. Interestingly, the basal (insulin-independent) interaction of the insulin receptor with PTP1B was much weaker with a soluble form of the tyrosine-phosphatase than with the endoplasmic reticulum (ER)-targeted form. Inhibition of insulin-receptor processing using tunicamycin suggests that the basal interaction occurs during insulin-receptor biosynthesis in the ER. Therefore, localization of PTP1B in this compartment might be important for the regulation of insulin receptors during their biosynthesis.
Figures



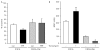

References
-
- Bandyopadhyay D., Kusari A., Kenner K.A., Liu F., Chernoff J., Gustafson T.A. & Kusari J. (1997) Protein-tyrosine phosphatase 1B complexes with the insulin receptor in vivo and is tyrosine-phosphorylated in the presence of insulin. J. Biol. Chem., 272, 1639–1645. - PubMed
-
- Boute N., Pernet K. & Issad T. (2001) Monitoring the activation state of the insulin receptor using bioluminescence resonance energy transfer. Mol. Pharmacol., 60, 640–645. - PubMed
-
- Boute N., Jockers R. & Issad T. (2002) The use of resonance energy transfer in high-throughput screening: BRET versus FRET. Trends Pharmacol. Sci., 23, 351–354. - PubMed
-
- Burgess J.W., Wada I., Ling N., Khan M.N., Bergeron J.J. & Posner B.I. (1992) Decrease in betasubunit phosphotyrosine correlates with internalization and activation of the endosomal insulin receptor kinase. J. Biol. Chem., 267, 10077–10086. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

