North American clinical experience with the EKOS MicroLysUS infusion catheter for the treatment of embolic stroke
- PMID: 12637311
- PMCID: PMC7973622
North American clinical experience with the EKOS MicroLysUS infusion catheter for the treatment of embolic stroke
Abstract
Background and purpose: We present early experience with the EKOS MicroLysUS infusion catheter for acute embolic stroke treatment in North America. This study was designed to demonstrate the safety of the device and to determine if sonography accelerates thrombolysis and improves clinical outcomes.
Methods: Fourteen patients aged 40-77 years with anterior- or posterior-circulation occlusion presented with cerebral ischemia 3-6 or 4-13 hours after symptom onset, respectively. Patients were treated with the catheter and simultaneous intraarterial thrombolysis. Procedural and clinical information, including time to lysis, degree of recanalization, National Institute of Health Stroke Scale (NIHSS) score, and modified Rankin Scale (mRS) score was recorded before treatment and afterward (immediately and at 24 hours, 1 week, 1 month, and 3 months).
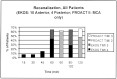
Results: Ten patients presented with acute anterior-circulation emboli; four patients, with posterior-circulation emboli (NIHSS score, 9-23 [mean. 18.2] and 11-27 [mean, 18.75], respectively). Three deaths occurred at 24 hours: two from hemorrhage and one from cerebral swelling. Deaths also occurred at 1 week and 1 month after treatment. Thrombolysis in Myocardial Ischemia grade 2-3 flow was achieved in eight patients in the first hour. Average time to recanalization was 46 minutes. Mean NIHSS scores in eight of nine survivors at 90 days were 5 in the anterior-circulation group and 3 in the posterior-circulation group; mean mRS scores at 90 days were 2 and 3, respectively. No catheter-related adverse events occurred.
Conclusion: Use of the EKOS MicroLysUS infusion catheter is feasible in the treatment of acute ischemic stroke. Further studies to evaluate its efficacy are warranted.
Figures
References
-
- Tissue plasminogen activator for acute ischemic stroke: The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. N Engl J Med 1995;333:1581–1587 - PubMed
-
- Dobkin B. The economic impact of stroke. Neurology 1995;45(suppl 1):S6–S9 - PubMed
-
- Del Zoppo GJ, Higashida RT, Furlan AJ, et al. PROACT: a phase II randomized trial of recombinant pro-urokinase by direct arterial delivery in acute middle cerebral artery stroke. Stroke 1998;29:4–11 - PubMed
-
- Furlan AJ, Higashida RT, Wechsler L, et al. Intra-arterial prourokinase for acute ischemic stroke: the PROACT II study—a randomized controlled trial. JAMA 1999;282:2003–2011 - PubMed
-
- Lewandowski CA, Frankel M, Tomsick TA, et al. Combined intravenous and intra-arterial r-TPA versus intra-arterial therapy of acute ischemic stroke: Emergency Management of Stroke (EMS) Bridging Trial. Stroke 1999;30:2598–2605 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources