Measles virus infection results in suppression of both innate and adaptive immune responses to secondary bacterial infection
- PMID: 12639986
- PMCID: PMC153759
- DOI: 10.1172/JCI13603
Measles virus infection results in suppression of both innate and adaptive immune responses to secondary bacterial infection
Abstract
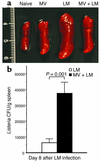
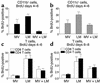
Among infectious agents, measles virus (MV) remains a scourge responsible for 1 million deaths per year and is a leading cause of childhood deaths in developing countries. Although MV infection itself is not commonly lethal, MV-induced suppression of the immune system results in a greatly increased susceptibility to opportunistic bacterial infections that are largely responsible for the morbidity and mortality associated with this disease. Despite its clinical importance, the underlying mechanisms of MV-induced immunosuppression remain unresolved. To begin to understand the basis of increased susceptibility to bacterial infections during MV infection, we inoculated transgenic mice expressing the MV receptor, CD46, with MV and Listeria monocytogenes. We found that MV-infected mice were more susceptible to infection with Listeria and that this corresponded with significantly decreased numbers of macrophages and neutrophils in the spleen and substantial defects in IFN-gamma production by CD4(+) T cells. The reduction in CD11b(+) macrophages and IFN-gamma-producing T cells was due to reduced proliferative expansion and not to enhanced apoptosis or to altered distribution of these cells between spleen, blood, and the lymphatic system. These results document that MV infection can suppress both innate and adaptive immune responses and lead to increased susceptibility to bacterial infection.
Figures
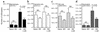
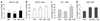

References
-
- Markowitz, L., and Katz, S. 1994. In Vaccines. S. Plotkin and B. Mortimer, editors. W.B. Saunders Co. Philadelphia, Pennsylvania, USA. 229–276.
-
- Griffin, D.E., and Bellini, W.J., editors. 1996. Measles virus. Lippincott-Raven. Philadelphia, Pennsylvania, USA. 1267–1312.
-
- Murray CJ, Lopez AD. Mortality by cause for eight regions of the world: Global Burden of Disease Study. Lancet. 1997;349:1269–1276. - PubMed
-
- Oldstone, M.B.A. 1998. Viruses, plagues, and history. Oxford University Press. New York, New York, USA. 73–89.
-
- Von Pirquet C. Das verhalten der kutanen tuberculinreaktion wahrend der masern. Dtsch. Med. Wochenschr. 1908;30:1297–1300.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

