Acceleration of type 1 diabetes mellitus in proinsulin 2-deficient NOD mice
- PMID: 12639991
- PMCID: PMC153768
- DOI: 10.1172/JCI16584
Acceleration of type 1 diabetes mellitus in proinsulin 2-deficient NOD mice
Abstract
Accumulating evidence favors a role for proinsulin as a key autoantigen in diabetes. In the mouse, two proinsulin isoforms coexist. Most studies point to proinsulin 2 as the major isoform recognized by T cells in the NOD mouse. We studied mice in which a null proinsulin 2 mutation was transferred from proinsulin 2-deficient 129 mice onto the NOD background along with 16 genetic markers (including I-A(g7) MHC molecule) associated with diabetes. Intercross mice from the fourth backcross generation showed that proinsulin 2(-/-) mice develop accelerated insulitis and diabetes. The high prevalence of anti-insulin autoantibodies in proinsulin 2(-/-) mice indicates that diabetes acceleration relates to altered recognition of proinsulin. The prevalence of anti-glutamic acid decarboxylase autoantibodies and of sialitis is not increased in proinsulin 2(-/-) mice. We give evidence that proinsulin 2 expression leads to silencing of T cells specific for an epitope shared by proinsulin 1 and proinsulin 2. In the human, alleles located in the VNTR region flanking the insulin gene control beta cell response to glucose and proinsulin expression in the thymus and are key determinants of diabetes susceptibility. Proinsulin 2(-/-) NOD mice provide a model to study the role of thymic expression of insulin in susceptibility to diabetes.
Figures


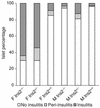

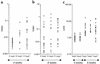

References
-
- Tisch R, et al. Immune response to glutamic acid decarboxylase correlates with insulitis in non-obese diabetic mice. Nature. 1993;366:72–75. - PubMed
-
- Palmer JP, et al. Insulin antibodies in insulin-dependent diabetics before insulin treatment. Science. 1983;222:1337–1339. - PubMed
-
- Kuglin B, Gries FA, Kolb H. Evidence of IgG autoantibodies against human proinsulin in patients with IDDM before insulin treatment. Diabetes. 1988;37:130–132. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

