Human phospholamban null results in lethal dilated cardiomyopathy revealing a critical difference between mouse and human
- PMID: 12639993
- PMCID: PMC153772
- DOI: 10.1172/JCI17892
Human phospholamban null results in lethal dilated cardiomyopathy revealing a critical difference between mouse and human
Abstract
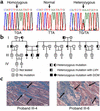
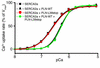
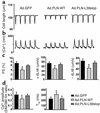
In human disease and experimental animal models, depressed Ca(2+) handling in failing cardiomyocytes is widely attributed to impaired sarcoplasmic reticulum (SR) function. In mice, disruption of the PLN gene encoding phospholamban (PLN) or expression of dominant-negative PLN mutants enhances SR and cardiac function, but effects of PLN mutations in humans are unknown. Here, a T116G point mutation, substituting a termination codon for Leu-39 (L39stop), was identified in two families with hereditary heart failure. The heterozygous individuals exhibited hypertrophy without diminished contractile performance. Strikingly, both individuals homozygous for L39stop developed dilated cardiomyopathy and heart failure, requiring cardiac transplantation at ages 16 and 27. An over 50% reduction in PLN mRNA and no detectable PLN protein were noted in one explanted heart. The expression of recombinant PLN-L39stop in human embryonic kidney (HEK) 293 cells and adult rat cardiomyocytes showed no PLN inhibition of SR Ca(2+)-ATPase and the virtual absence of stable PLN expression; where PLN was expressed, it was misrouted to the cytosol or plasma membrane. These findings describe a naturally-occurring loss-of-function human PLN mutation (PLN null). In contrast to reported benefits of PLN ablation in mouse heart failure, humans lacking PLN develop lethal dilated cardiomyopathy.
Figures



Comment in
-
The challenge of molecular medicine: complexity versus Occam's razor.J Clin Invest. 2003 Mar;111(6):801-3. doi: 10.1172/JCI18153. J Clin Invest. 2003. PMID: 12639985 Free PMC article. No abstract available.
References
-
- Cohn JN. Current concepts in the treatment of congestive heart failure. Cardiology. 1997;88(Suppl. 2):2–6. - PubMed
-
- Ho KK, Pinsky JL, Kannel WB, Levy D. The epidemiology of heart failure: the Framingham Study. J. Am. Coll. Cardiol. 1993;22:6A–13A. - PubMed
-
- Hasenfuss G. Alterations of calcium-regulatory proteins in heart failure. Cardiovasc. Res. 1998;37:279–289. - PubMed
-
- Beuckelmann DJ, Nabauer M, Erdmann E. Intracellular calcium handling in isolated ventricular myocytes from patients with terminal heart failure. Circulation. 1992;85:1046–1055. - PubMed
-
- Endo M. Calcium release from the sarcoplasmic reticulum. Physiol. Rev. 1977;57:71–108. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

