DNA damage is a novel response to sublytic complement C5b-9-induced injury in podocytes
- PMID: 12639994
- PMCID: PMC153762
- DOI: 10.1172/JCI15645
DNA damage is a novel response to sublytic complement C5b-9-induced injury in podocytes
Abstract
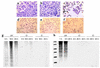
In response to Ab-complement-mediated injury, podocytes can undergo lysis, apoptosis, or, when exposed to sublytic (<5% lysis) amounts of C5b-9, become activated. Following the insertion of sublytic quantities of C5b-9, there is an increase in signaling pathways and growth factor synthesis and release of proteases, oxidants, and other molecules. Despite an increase in DNA synthesis, however, sublytic C5b-9 is associated with a delay in G(2)/M phase progression in podocytes. Here we induced sublytic C5b-9 injury in vitro by exposing cultured rat podocytes or differentiated postmitotic mouse podocytes to Ab and a complement source; we also studied the passive Heymann nephritis model of experimental membranous nephropathy in rats. A major finding was that sublytic C5b-9-induced injury caused an increase in DNA damage in podocytes both in vitro and in vivo. This was associated with an increase in protein levels for p53, the CDK inhibitor p21, growth-arrest DNA damage-45 (GADD45), and the checkpoint kinases-1 and -2. Sublytic C5b-9 increased extracellular signal-regulated kinase-1 and -2 (ERK-1 and -2), and inhibiting ERK-1 and -2 reduced the increase in p21 and GADD45 and augmented the DNA damage response to sublytic C5b-9-induced injury. These results show that sublytic C5b-9 induces DNA damage in vitro and in vivo and may explain why podocyte proliferation is limited following immune-mediated injury.
Figures
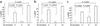




References
-
- Couser WG. Mediation of immune glomerular injury. J. Am. Soc. Nephrol. 1990;1:13–29. - PubMed
-
- Cybulsky AV, Monge JC, Papillon J, McTavish AJ. Complement C5b-9 activates cytosolic phospholipase A2 in glomerular epithelial cells. Am. J. Physiol. 1995;269:F739–F749. - PubMed
-
- Torbohm I, et al. C5b-8 and C5b-9 modulate the collagen release of human glomerular epithelial cells. Kidney Int. 1990;37:1098–1104. - PubMed
-
- Hansch GM, Seitz M, Betz M. Effect of the late complement components C5b-9 on human monocytes: release of prostanoids, oxygen radicals and of a factor inducing cell proliferation. Int. Arch. Allergy Appl. Immunol. 1987;82:317–320. - PubMed
-
- Lovett DH, Haensch G-M, Goppelt M, Resch KDG. Activation of glomerular mesangial cells by the terminal membrane attack complex of complement. J. Immunol. 1987;138:2413–2480. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

