Immunization with neuronal nicotinic acetylcholine receptor induces neurological autoimmune disease
- PMID: 12639997
- PMCID: PMC153771
- DOI: 10.1172/JCI17429
Immunization with neuronal nicotinic acetylcholine receptor induces neurological autoimmune disease
Abstract
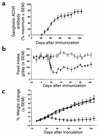
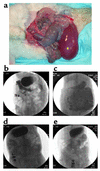
Neuronal nicotinic AChRs (nAChRs) are implicated in the pathogenesis of diverse neurological disorders and in the regulation of small-cell lung carcinoma growth. Twelve subunits have been identified in vertebrates, and mutations of one are recognized in a rare form of human epilepsy. Mice with genetically manipulated neuronal nAChR subunits exhibit behavioral or autonomic phenotypes. Here, we report the first model of an acquired neuronal nAChR disorder and evidence for its pertinence to paraneoplastic neurological autoimmunity. Rabbits immunized once with recombinant alpha3 subunit (residues 1-205) develop profound gastrointestinal hypomotility, dilated pupils with impaired light response, and grossly distended bladders. As in patients with idiopathic and paraneoplastic autoimmune autonomic neuropathy, the severity parallels serum levels of ganglionic nAChR autoantibody. Failure of neurotransmission through abdominal sympathetic ganglia, with retention of neuronal viability, confirms that the disorder is a postsynaptic channelopathy. In addition, we found ganglionic nAChR protein in small-cell carcinoma lines, identifying this cancer as a potential initiator of ganglionic nAChR autoimmunity. The data support our hypothesis that immune responses driven by distinct neuronal nAChR subtypes expressed in small-cell carcinomas account for several lung cancer-related paraneoplastic disorders affecting cholinergic systems, including autoimmune autonomic neuropathy, seizures, dementia, and movement disorders.
Figures


Comment in
-
Autonomic "myasthenia": the case for an autoimmune pathogenesis.J Clin Invest. 2003 Mar;111(6):797-9. doi: 10.1172/JCI18180. J Clin Invest. 2003. PMID: 12639983 Free PMC article. No abstract available.
References
-
- Musunuru K, Darnell RB. Review. Paraneoplastic neurologic disease antigens: RNA-binding proteins and signaling proteins in neuronal degeneration. Annu. Rev. Neurosci. 2001;24:239–262. - PubMed
-
- Steinman L, Martin R, Bernard C, Conlon P, Oksenberg JR. Multiple sclerosis: deeper understanding of its pathogenesis reveals new targets for therapy. Annu. Rev. Neurosci. 2002;25:491–505. - PubMed
-
- Willison H, Stoll G, Toyka KV, Berger T, Hartung HP. Autoimmunity and inflammation in the peripheral nervous system. Research update. Trends Neurosci. 2002;25:127–129. - PubMed
-
- Patrick J, Lindstrom J. Autoimmune response to acetylcholine receptor. Science. 1973;180:871–872. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

