Developmental changes in contractility and sarcomeric proteins from the early embryonic to the adult stage in the mouse heart
- PMID: 12640016
- PMCID: PMC2342849
- DOI: 10.1113/jphysiol.2002.036509
Developmental changes in contractility and sarcomeric proteins from the early embryonic to the adult stage in the mouse heart
Abstract
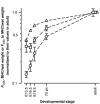
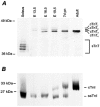
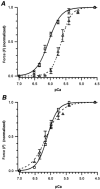
Developmental changes in force-generating capacity and Ca2+ sensitivity of contraction in murine hearts were correlated with changes in myosin heavy chain (MHC) and troponin (Tn) isoform expression, using Triton-skinned fibres. The maximum Ca2+-activated isometric force normalized to the cross-sectional area (FCSA) increased mainly during embryogenesis and continued to increase at a slower rate until adulthood. During prenatal development, FCSA increased about 5-fold from embryonic day (E)10.5 to E19.5, while the amount of MHC normalized to the amount of total protein remained constant (from E13.5 to E19.5). This suggests that the development of structural organization of the myofilaments during the embryonic and the fetal period may play an important role for the improvement of force generation. There was an overall decrease of 0.5 pCa units in the Ca2+ sensitivity of force generation from E13.5 to the adult, of which the main decrease (0.3 pCa units) occurred within a short time interval, between E19.5 and 7 days after birth (7 days pn). Densitometric analysis of SDS-PAGE and Western blots revealed that the major switches between troponin T (TnT) isoforms occur before E16.5, whereas the transition points of slow skeletal troponin I (ssTnI) to cardiac TnI (cTnI) and of beta-MHC to alpha-MHC both occur around birth, in temporal correlation with the main decrease in Ca2+ sensitivity. To test whether the changes in Ca2+ sensitivity are solely based on Tn, the native Tn complex was replaced in fibres from E19.5 and adult hearts with fast skeletal Tn complex (fsTn) purified from rabbit skeletal muscle. The difference in pre-replacement values of pCa50 (-log([Ca2+] M-1)) required for half-maximum force development) between E19.5 (6.05 +/- 0.01) and adult fibres (5.64 +/- 0.04) was fully abolished after replacement with the exogenous skeletal Tn complex (pCa50 = 6.12 +/- 0.05 for both stages). This suggests that the major developmental changes in Ca2+ sensitivity of skinned murine myocardium originate primarily from the switch of ssTnI to cTnI.
Figures




References
-
- Agbulut O, Li Z, Mouly V, Butler-Browne GS. Analysis of skeletal and cardiac muscle from desmin knock-out and normal mice by high resolution separation of myosin heavy-chain isoforms. Biol Cell. 1996;88:131–135. - PubMed
-
- Anderson PA, Glick KL, Manring A, Crenshaw C., Jr Developmental changes in cardiac contractility in fetal and postnatal sheep: in vitro and in vivo. Am J Physiol Heart Circ Physiol. 1984;247:H371–379. - PubMed
-
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. - PubMed
-
- Cappelli V, Bottinelli R, Poggesi C, Moggio R, Reggiani C. Shortening velocity and myosin and myofibrillar ATPase activity related to myosin isoenzyme composition during postnatal development in rat myocardium. Circ Res. 1989;65:446–457. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

