Caveolin-induced activation of the phosphatidylinositol 3-kinase/Akt pathway increases arsenite cytotoxicity
- PMID: 12640124
- PMCID: PMC150728
- DOI: 10.1128/MCB.23.7.2407-2414.2003
Caveolin-induced activation of the phosphatidylinositol 3-kinase/Akt pathway increases arsenite cytotoxicity
Abstract
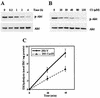
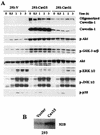
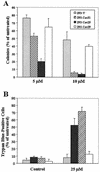
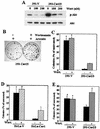
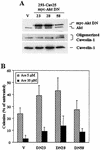
The inhibitory effect of caveolin on the cellular response to growth factor stimulation is well established. Given the significant overlap in signaling pathways involved in regulating cell proliferation and stress responsiveness, we hypothesized that caveolin would also affect a cell's ability to respond to environmental stress. Here we investigated the ability of caveolin-1 to modulate the cellular response to sodium arsenite and thereby alter survival of the human cell lines 293 and HeLa. Cells stably transfected with caveolin-1 were found to be much more sensitive to the toxic effects of sodium arsenite than either untransfected parental cells or parental cells transfected with an empty vector. Unexpectedly, the caveolin-overexpressing cells also exhibited a significant activation of the phosphatidylinositol 3-kinase (PI3K)/Akt pathway, which additional studies suggested was likely due to decreased neutral sphingomyelinase activity and ceramide synthesis. In contrast to its extensively documented antiapoptotic influence, the elevated activity of Akt appears to be important in sensitizing caveolin-expressing cells to arsenite-induced toxicity, as both pretreatment of cells with the PI3K inhibitor wortmannin and overexpression of a dominant-negative Akt mutant markedly improved the survival of arsenite-treated cells. This death-promoting influence of the PI3K/Akt pathway in caveolin-overexpressing cells appeared not to be unique to sodium arsenite, as wortmannin pretreatment also resulted in increased survival in the presence of H(2)O(2). In summary, our results indicate that caveolin-induced upregulation of the PI3K/Akt signaling pathway, which appears to be a death signal in the presence of arsenite and H(2)O(2), sensitizes cells to environmental stress.
Figures



References
-
- Anderson, R. G. W. 1998. The caveolae membrane system. Annu. Rev. Biochem. 67:199-225. - PubMed
-
- Bagla, P., and J. Kaiser. 1996. India's spreading health crisis draws global arsenic experts. Science 274:174-175. - PubMed
-
- Barchowsky, A., E. J. Dudek, M. D. Treadwell, and K. E. Wetterhan. 1996. Arsenic induces oxidant stress and NF-kappa B activation in cultured aortic endothelial cells. Free Radic. Biol. Med. 21:783-790. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
