Myocardin is a critical serum response factor cofactor in the transcriptional program regulating smooth muscle cell differentiation
- PMID: 12640126
- PMCID: PMC150745
- DOI: 10.1128/MCB.23.7.2425-2437.2003
Myocardin is a critical serum response factor cofactor in the transcriptional program regulating smooth muscle cell differentiation
Abstract
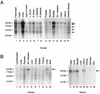
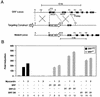
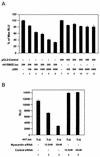
The SAP family transcription factor myocardin functionally synergizes with serum response factor (SRF) and plays an important role in cardiac development. To determine the function of myocardin in the smooth muscle cell (SMC) lineage, we mapped the pattern of myocardin gene expression and examined the molecular mechanisms underlying transcriptional activity of myocardin in SMCs and embryonic stem (ES) cells. The human and murine myocardin genes were expressed in vascular and visceral SMCs at levels equivalent to or exceeding those observed in the heart. During embryonic development, the myocardin gene was expressed abundantly in a precise, developmentally regulated pattern in SMCs. Forced expression of myocardin transactivated multiple SMC-specific transcriptional regulatory elements in non-SMCs. By contrast, myocardin-induced transactivation was not observed in SRF(-/-) ES cells but could be rescued by forced expression of SRF or the SRF DNA-binding domain. Furthermore, expression of a dominant-negative myocardin mutant protein or small-interfering-RNA-induced myocardin knockdown significantly reduced SM22 alpha promoter activity in SMCs. Most importantly, forced expression of myocardin activated expression of the SM22 alpha, smooth muscle alpha-actin, and calponin-h1 genes in undifferentiated mouse ES cells. Taken together, these data demonstrate that myocardin plays an important role in the SRF-dependent transcriptional program that regulates SMC development and differentiation.
Figures





References
-
- Aravind, L., and E. V. Koonin. 2000. SAP—a putative DNA-binding motif involved in chromosomal organization. Trends Biochem. Sci. 25:112-114. - PubMed
-
- Chang, P. S., L. Li, J. McAnally, and E. N. Olson. 2001. Muscle specificity encoded by specific serum response factor-binding sites. J. Biol. Chem. 276:17206-17212. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
