Detection of a functional hybrid receptor gammac/GM-CSFRbeta in human hematopoietic CD34+ cells
- PMID: 12642604
- PMCID: PMC2193857
- DOI: 10.1084/jem.20020150
Detection of a functional hybrid receptor gammac/GM-CSFRbeta in human hematopoietic CD34+ cells
Abstract
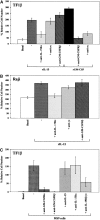
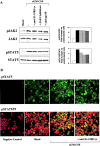
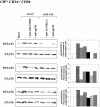
A functional hybrid receptor associating the common gamma chain (gammac) with the granulocyte/macrophage colony-stimulating factor receptor beta (GM-CSFRbeta) chain is found in mobilized human peripheral blood (MPB) CD34+ hematopoietic progenitors, SCF/Flt3-L primed cord blood (CB) precursors (CBPr CD34+/CD56-), and CD34+ myeloid cell lines, but not in normal natural killer (NK) cells, the cytolytic NK-L cell line or nonhematopoietic cells. We demonstrated, using CD34+ TF1beta cells, which express an interleukin (IL)-15Ralpha/beta/gammac receptor, that within the hybrid receptor, the GM-CSFRbeta chain inhibits the IL-15-triggered gammac/JAK3-specific signaling controlling TF1beta cell proliferation. However, the gammac chain is part of a functional GM-CSFR, activating GM-CSF-dependent STAT5 nuclear translocation and the proliferation of TF1beta cells. The hybrid receptor is functional in normal hematopoietic progenitors in which both subunits control STAT5 activation. Finally, the parental TF1 cell line, which lacks the IL-15Rbeta chain, nevertheless expresses both a functional hybrid receptor that controls JAK3 phosphorylation and a novel IL-15alpha/gammac/TRAF2 complex that triggers nuclear factor kappaB activation. The lineage-dependent distribution and function of these receptors suggest that they are involved in hematopoiesis because they modify transduction pathways that play a major role in the differentiation of hematopoietic progenitors.
Figures



Similar articles
-
Altered natural killer cell differentiation in CD34+ progenitors from chronic myeloid leukemia patients.Oncogene. 2000 May 25;19(23):2758-66. doi: 10.1038/sj.onc.1203584. Oncogene. 2000. PMID: 10851076
-
Interleukin-11 stimulates the proliferation of human hematopoietic CD34+ and CD34+CD33-DR- cells and synergizes with stem cell factor, interleukin-3, and granulocyte-macrophage colony-stimulating factor.Exp Hematol. 1993 Dec;21(13):1668-72. Exp Hematol. 1993. PMID: 7694867
-
Expression of FLT3 receptor and response to FLT3 ligand by leukemic cells.Leukemia. 1996 Apr;10(4):588-99. Leukemia. 1996. PMID: 8618433 Review.
-
Stem cell factor (c-kit ligand) enhances the interleukin-9-dependent proliferation of human CD34+ and CD34+CD33-DR- cells.Exp Hematol. 1994 Aug;22(9):919-23. Exp Hematol. 1994. PMID: 7520394
-
The biology of IL-15: implications for cancer therapy and the treatment of autoimmune disorders.J Investig Dermatol Symp Proc. 2013 Dec;16(1):S28-30. doi: 10.1038/jidsymp.2013.8. J Investig Dermatol Symp Proc. 2013. PMID: 24326545 Review.
Cited by
-
Recombinant IL-7/HGFβ efficiently induces transplantable murine hematopoietic stem cells.J Clin Invest. 2012 Oct;122(10):3552-62. doi: 10.1172/JCI46055. Epub 2012 Sep 17. J Clin Invest. 2012. PMID: 22996694 Free PMC article.
-
STAT3- and STAT5-dependent pathways competitively regulate the pan-differentiation of CD34pos cells into tumor-competent dendritic cells.Blood. 2008 Sep 1;112(5):1832-43. doi: 10.1182/blood-2007-12-130138. Epub 2008 Jun 24. Blood. 2008. PMID: 18577706 Free PMC article.
-
Crosstalk between IL-15Rα+ tumor-associated macrophages and breast cancer cells reduces CD8+ T cell recruitment.Cancer Commun (Lond). 2022 Jun;42(6):536-557. doi: 10.1002/cac2.12311. Epub 2022 May 25. Cancer Commun (Lond). 2022. PMID: 35615815 Free PMC article.
-
Irradiation-induced up-regulation of HLA-E on macrovascular endothelial cells confers protection against killing by activated natural killer cells.PLoS One. 2010 Dec 16;5(12):e15339. doi: 10.1371/journal.pone.0015339. PLoS One. 2010. PMID: 21179573 Free PMC article.
-
IL2RG, identified as overexpressed by RNA-seq profiling of pancreatic intraepithelial neoplasia, mediates pancreatic cancer growth.Oncotarget. 2017 Aug 3;8(48):83370-83383. doi: 10.18632/oncotarget.19848. eCollection 2017 Oct 13. Oncotarget. 2017. PMID: 29137350 Free PMC article.
References
-
- Ratajczak, M.Z., and A.M. Gewirtz. 1995. The biology of hemopoietic stem cells. Semin. Oncol. 22:210–217. - PubMed
-
- Sharkis, S.J., C. Cremo, M.I. Collector, S.J. Noga, and A.D. Donnenberg. 1986. Thymic regulation of hematopoiesis: isolation of helper and suppressor populations using counterflow centrifugal elutriation. Blood. 68:787–789. - PubMed
-
- Majka, M., A. Janowska-Wieczorek, J. Ratajczak, K. Ehrenman, Z. Pietrzkowski, M.A. Kowalska, A.M. Gewirtz, S.G. Emerson, and M.Z. Ratajczak. 2001. Numerous growth factors, cytokines, and chemokines are secreted by human CD34(+) cells, myeloblasts, erythroblasts, and megakaryoblasts and regulate normal hematopoiesis in an autocrine/paracrine manner. Blood. 97:3075–3085. - PubMed
-
- Shibuya, A., K. Taguchi, H. Kojima, and T. Abe. 1991. Inhibitory effect of granulocyte-macrophage colony-stimulating factor therapy on the generation of natural killer cells. Blood. 78:3241–324. - PubMed
-
- Miller, J.S., F. Prosper, and V. McCullar. 1997. Natural killer (NK) cells are functionally abnormal and NK cell progenitors are diminished in granulocyte colony-stimulating factor-mobilized peripheral blood. Blood. 90:3098–3105. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

