Polarized growth and organelle segregation in yeast: the tracks, motors, and receptors
- PMID: 12642608
- PMCID: PMC2173777
- DOI: 10.1083/jcb.200301035
Polarized growth and organelle segregation in yeast: the tracks, motors, and receptors
Abstract
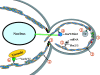
In yeast, growth and organelle segregation requires formin-dependent assembly of polarized actin cables. These tracks are used by myosin Vs to deliver secretory vesicles for cell growth, organelles for their segregation, and mRNA for fate determination. Several specific receptors have been identified that interact with the cargo-binding tails of the myosin Vs. A recent study implicates specific degradation in the bud of the vacuolar receptor, Vac17, as a mechanism for cell cycle-regulated segregation of this organelle.
Figures
References
-
- Alberts, A.S. 2001. Identification of a carboxyl-terminal diaphanous-related formin homology protein autoregulatory domain. J. Biol. Chem. 276:2824–2830. - PubMed
-
- Beach, D.L., J. Thibodeaux, P. Maddox, E. Yeh, and K. Bloom. 2000. The role of the proteins Kar9 and Myo2 in orienting the mitotic spindle of budding yeast. Curr. Biol. 10:1497–1506. - PubMed
-
- Bobola, N., R.P. Jansen, T.H. Shin, and K. Nasmyth. 1996. Asymmetric accumulation of Ash1p in postanaphase nuclei depends on a myosin and restricts yeast mating-type switching to mother cells. Cell. 84:699–709. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

