The RasGAP-associated endoribonuclease G3BP assembles stress granules
- PMID: 12642610
- PMCID: PMC2173781
- DOI: 10.1083/jcb.200212128
The RasGAP-associated endoribonuclease G3BP assembles stress granules
Retraction in
-
Retract and Replace: The RasGAP-associated endoribonuclease G3BP assembles stress granules.J Cell Biol. 2023 Nov 6;222(11):e20021212808022023r. doi: 10.1083/jcb.20021212808022023r. Epub 2023 Sep 6. J Cell Biol. 2023. PMID: 37672658 Free PMC article. No abstract available.
Abstract
Stress granules (SGs) are formed in the cytoplasm in response to various toxic agents, and are believed to play a critical role in the regulation of mRNA metabolism during stress. In SGs, mRNAs are stored in an abortive translation initiation complex that can be routed to either translation initiation or degradation. Here, we show that G3BP, a phosphorylation-dependent endoribonuclease that interacts with RasGAP, is recruited to SGs in cells exposed to arsenite. G3BP may thus determine the fate of mRNAs during cellular stress. Remarkably, SG assembly can be either dominantly induced by G3BP overexpression, or on the contrary, inhibited by expressing a central domain of G3BP. This region binds RasGAP and contains serine 149, whose dephosphorylation is induced by arsenite treatment. Critically, a phosphomimetic mutant (S149E) fails to oligomerize and to assemble SGs, whereas a nonphosphorylatable G3BP mutant (S149A) does both. These results suggest that G3BP is an effector of SG assembly, and that Ras signaling contributes to this process by regulating G3BP dephosphorylation.
Figures

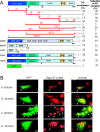
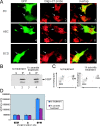
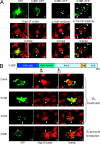

Comment in
-
Phosphorylation of G3BP1-S149 does not influence stress granule assembly.J Cell Biol. 2019 Jul 1;218(7):2425-2432. doi: 10.1083/jcb.201801214. Epub 2019 Jun 6. J Cell Biol. 2019. PMID: 31171631 Free PMC article.
-
Reply to "Phosphorylation of G3BP1-S149 does not influence stress granule assembly".J Cell Biol. 2019 Jul 1;218(7):2433-2434. doi: 10.1083/jcb.201905105. Epub 2019 Jun 6. J Cell Biol. 2019. PMID: 31171633 Free PMC article.
References
-
- Bullock, T.L., W.D. Clarkson, H.M. Kent, and M. Stewart. 1996. The 1.6 angstroms resolution crystal structure of nuclear transport factor 2 (NTF2). J. Mol. Biol. 260:422–431. - PubMed
-
- Gallouzi, I.E., F. Parker, K. Chebli, F. Maurier, E. Labourier, I. Barlat, J.P. Capony, B. Tocque, and J. Tazi. 1998. A novel phosphorylation-dependent RNase activity of GAP-SH3 binding protein: a potential link between signal transduction and RNA stability. Mol. Cell. Biol. 18:3956–3965. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

