PAR-dependent and geometry-dependent mechanisms of spindle positioning
- PMID: 12642612
- PMCID: PMC2173762
- DOI: 10.1083/jcb.200209079
PAR-dependent and geometry-dependent mechanisms of spindle positioning
Abstract
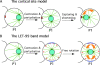
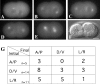
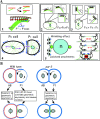
During intrinsically asymmetric division, the spindle is oriented onto a polarized axis specified by a group of conserved PAR proteins. Extrinsic geometric asymmetry generated by cell shape also affects spindle orientation in some systems, but how intrinsic and extrinsic mechanisms coexist without interfering with each other is unknown. In some asymmetrically dividing cells of the wild-type Caenorhabditis elegans embryo, nuclear rotation directed toward the anterior cortex orients the forming spindle. We find that in such cells, a PAR-dependent mechanism dominates and causes rotation onto the polarized axis, regardless of cell shape. However, when geometric asymmetry is removed, free nuclear rotation in the center of the cell is observed, indicating that the anterior-directed nature of rotation in unaltered embryos is an effect of cell shape. This free rotation is inconsistent with the prevailing model for nuclear rotation, the specialized cortical site model. In contrast, in par-3 mutant embryos, a geometry-dependent mechanism becomes active and causes directed nuclear rotation. These results lead to the model that in wild-type embryos both PAR-3 and PAR-2 are essential for nuclear rotation in asymmetrically dividing cells, but that PAR-3 inhibits geometry-dependent rotation in nonpolarized cells, thus preventing cell shape from interfering with spindle orientation.
Figures



References
-
- Bowerman, B., and C.A. Shelton. 1999. Cell polarity in the early Caenorhabditis elegans embryo. Curr. Opin. Genet. Dev. 9:390–395. - PubMed
-
- Boyd, L., S. Guo, D. Levitan, D.T. Stinchcomb, and K.J. Kemphues. 1996. PAR-2 is asymmetrically distributed and promotes association of P granules and PAR-1 with the cortex in C. elegans embryos. Development. 122:3075–3084. - PubMed

