Bifunctional antisense oligonucleotides provide a trans-acting splicing enhancer that stimulates SMN2 gene expression in patient fibroblasts
- PMID: 12642665
- PMCID: PMC153057
- DOI: 10.1073/pnas.0633863100
Bifunctional antisense oligonucleotides provide a trans-acting splicing enhancer that stimulates SMN2 gene expression in patient fibroblasts
Abstract
The multiplicity of proteins compared with genes in mammals owes much to alternative splicing. Splicing signals are so subtle and complex that small perturbations may allow the production of new mRNA variants. However, the flexibility of splicing can also be a liability, and several genetic diseases result from single-base changes that cause exons to be skipped during splicing. Conventional oligonucleotide strategies can block reactions but cannot restore splicing. We describe here a method by which the use of a defective exon was restored. Spinal muscular atrophy (SMA) results from mutations of the Survival Motor Neuron (SMN) gene. Mutations of SMN1 cause SMA, whereas SMN2 acts as a modifying gene. The two genes undergo alternative splicing with SMN1, producing an abundance of full-length mRNA transcripts, whereas SMN2 predominantly produces exon 7-deleted transcripts. This discrepancy is because of a single nucleotide difference in SMN2 exon 7, which disrupts an exonic splicing enhancer containing an SF2ASF binding site. We have designed oligoribonucleotides that are complementary to exon 7 and contain exonic splicing enhancer motifs to provide trans-acting enhancers. These tailed oligoribonucleotides increased SMN2 exon 7 splicing in vitro and rescued the incorporation of SMN2 exon 7 in SMA patient fibroblasts. This treatment also resulted in the partial restoration of gems, intranuclear structures containing SMN protein that are severely reduced in patients with SMA. The use of tailed antisense oligonucleotides to recruit positively acting factors to stimulate a splicing reaction may have therapeutic applications for genetic disorders, such as SMA, in which splicing patterns are altered.
Figures


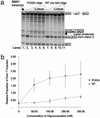

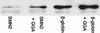
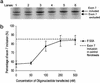

Comment in
-
Putting some spine into alternative splicing.Trends Biotechnol. 2003 Aug;21(8):328-30. doi: 10.1016/S0167-7799(03)00168-9. Trends Biotechnol. 2003. PMID: 12902166
References
-
- Dubowitz V. Muscle Disorders in Childhood. 2nd Ed. London: Saunders; 1995.
-
- Lefebvre S, Burglen L, Reboullet S, Clermont O, Burlet P, Viollet L, Benichou B, Cruaud C, Millasseau P, Zeviani M. Cell. 1995;80:155–165. - PubMed
-
- Taylor J E, Thomas N H, Lewis C M, Abbs S J, Rodrigues N R, Davies K E, Mathew C G. Eur J Hum Genet. 1998;6:467–474. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

