The role of disulfide bonds in the assembly and function of MD-2
- PMID: 12642668
- PMCID: PMC153023
- DOI: 10.1073/pnas.0630495100
The role of disulfide bonds in the assembly and function of MD-2
Abstract
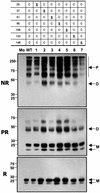
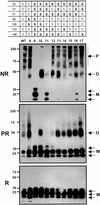
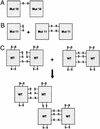
MD-2 is a secreted glycoprotein that binds to the extracellular domain of Toll-like receptor 4 (TLR4) and is required for the activation of TLR4 by lipopolysaccharide (LPS). The protein contains seven Cys residues and consists of a heterogeneous collection of disulfide-linked oligomers. To investigate the role of sulfhydryls in MD-2 structure and function, we created 17 single and multiple Cys substitution mutants. All of the MD-2 mutant proteins, including one totally lacking Cys residues, were secreted and stable. SDSPAGE analyses indicated that most Cys residues could participate in oligomer formation and that no single Cys residue was required for oligomerization. Of the single Cys substitutions, only C95S and C105S failed to confer LPS responsiveness on TLR4 when mutant and TLR4 were cotransfected into cells expressing an NF-kappaB reporter plasmid. Surprisingly, substitution of both C95 and C105 partially restored activity. Structural analyses revealed that C95 and C105 formed an intrachain disulfide bond, whereas C95 by itself produced an inactive dimer. In contrast to the cotransfection experiments, only WT MD-2 conferred responsiveness to LPS when secreted proteins were added directly to TLR4 reporter cells. Our data are consistent with a model in which most, possibly all sulfhydryls lie on the surface of a stable MD-2 core structure where they form both intra- and interchain disulfide bridges. These disulfide bonds produce a heterogeneous array of oligomers, including some species that can form an active complex with TLR4.
Figures

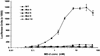
References
-
- Akira S, Takeda K, Kaisho T. Nat Immunol. 2001;2:675–680. - PubMed
-
- Imler J L, Hoffmann J A. Trends Cell Biol. 2001;11:304–311. - PubMed
-
- Medzhitov R. Nat Rev Immunol. 2001;1:135–145. - PubMed
-
- Dziarski R, Gupta D. J Endotoxin Res. 2000;6:401–405. - PubMed
-
- Poltorak A, He X, Smirnova I, Liu M Y, Huffel C V, Du X, Birdwell D, Alejos E, Silva M, Galanos C, et al. Science. 1998;282:2085–2088. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

