OmcB, a c-type polyheme cytochrome, involved in Fe(III) reduction in Geobacter sulfurreducens
- PMID: 12644478
- PMCID: PMC151516
- DOI: 10.1128/JB.185.7.2096-2103.2003
OmcB, a c-type polyheme cytochrome, involved in Fe(III) reduction in Geobacter sulfurreducens
Abstract
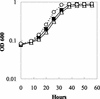
Microorganisms in the family Geobacteraceae are the predominant Fe(III)-reducing microorganisms in a variety of subsurface environments in which Fe(III) reduction is an important process, but little is known about the mechanisms for electron transport to Fe(III) in these organisms. The Geobacter sulfurreducens genome was found to contain a 10-kb chromosomal duplication consisting of two tandem three-gene clusters. The last genes of the two clusters, designated omcB and omcC, encode putative outer membrane polyheme c-type cytochromes which are 79% identical. The role of the omcB and omcC genes in Fe(III) reduction in G. sulfurreducens was investigated. OmcB and OmcC were both expressed during growth with acetate as the electron donor and either fumarate or Fe(III) as the electron acceptor. OmcB was ca. twofold more abundant under both conditions. Disrupting omcB or omcC by gene replacement had no impact on growth with fumarate. However, the OmcB-deficient mutant was greatly impaired in its ability to reduce Fe(III) both in cell suspensions and under growth conditions. In contrast, the ability of the OmcC-deficient mutant to reduce Fe(III) was similar to that of the wild type. When omcB was reintroduced into the OmcB-deficient mutant, the capacity for Fe(III) reduction was restored in proportion to the level of OmcB production. These results indicate that OmcB, but not OmcC, has a major role in electron transport to Fe(III) and suggest that electron transport to the outer membrane is an important feature in Fe(III) reduction in this organism.
Figures


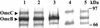
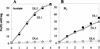
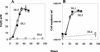
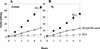
References
-
- Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl (ed.). 1999. Current protocols in molecular biology, vol. 2. John Wiley & Sons, Inc., New York, N.Y.
-
- Beliaev, A., D. Saffarini, J. McLaughlin, and D. Hunnicutt. 2001. MtrC, an outer membrane decahaem c cytochrome required for metal reduction in Shewanella putrefaciens MR-1. Mol. Microbiol. 39:722-730. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

