Expression of a GALACTINOL SYNTHASE gene in tomato seeds is up-regulated before maturation desiccation and again after imbibition whenever radicle protrusion is prevented
- PMID: 12644684
- PMCID: PMC166894
- DOI: 10.1104/pp.016386
Expression of a GALACTINOL SYNTHASE gene in tomato seeds is up-regulated before maturation desiccation and again after imbibition whenever radicle protrusion is prevented
Abstract
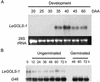
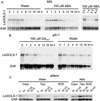
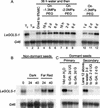
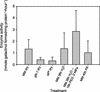
Raffinose family oligosaccharides (RFOs) have been implicated in mitigating the effects of environmental stresses on plants. In seeds, proposed roles for RFOs include protecting cellular integrity during desiccation and/or imbibition, extending longevity in the dehydrated state, and providing substrates for energy generation during germination. A gene encoding galactinol synthase (GOLS), the first committed enzyme in the biosynthesis of RFOs, was cloned from tomato (Lycopersicon esculentum Mill. cv Moneymaker) seeds, and its expression was characterized in tomato seeds and seedlings. GOLS (LeGOLS-1) mRNA accumulated in developing tomato seeds concomitant with maximum dry weight deposition and the acquisition of desiccation tolerance. LeGOLS-1 mRNA was present in mature, desiccated seeds but declined within 8 h of imbibition in wild-type seeds. However, LeGOLS-1 mRNA accumulated again in imbibed seeds prevented from completing germination by dormancy or water deficit. Gibberellin-deficient (gib-1) seeds maintained LeGOLS-1 mRNA amounts after imbibition unless supplied with gibberellin, whereas abscisic acid (ABA) did not prevent the loss of LeGOLS-1 mRNA from wild-type seeds. The presence of LeGOLS-1 mRNA in ABA-deficient (sitiens) tomato seeds indicated that wild-type amounts of ABA are not necessary for its accumulation during seed development. In all cases, LeGOLS-1 mRNA was most prevalent in the radicle tip. LeGOLS-1 mRNA accumulation was induced by dehydration but not by cold in germinating seeds, whereas both stresses induced LeGOLS-1 mRNA accumulation in seedling leaves. The physiological implications of LeGOLS-1 expression patterns in seeds and leaves are discussed in light of the hypothesized role of RFOs in plant stress tolerance.
Figures



References
-
- Amuti KS, Pollard CJ. Soluble carbohydrates of dry and developing seeds. Phytochemistry. 1977;16:529–532.
-
- Anderson CM, Kohorn BD. Inactivation of Arabidopsis SIP1 leads to reduced levels of sugars and drought tolerance. J Plant Physiol. 2001;158:1215–1219.
-
- Ashworth EN, Stirm VE, Volenec JJ. Seasonal variations in soluble sugars and starch within woody stems of Cornus sericea L. Tree Physiol. 1993;13:379–388. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources

