Carbon export from arbuscular mycorrhizal roots involves the translocation of carbohydrate as well as lipid
- PMID: 12644699
- PMCID: PMC166909
- DOI: 10.1104/pp.102.007765
Carbon export from arbuscular mycorrhizal roots involves the translocation of carbohydrate as well as lipid
Abstract
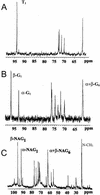
Arbuscular mycorrhizal (AM) fungi take up photosynthetically fixed carbon from plant roots and translocate it to their external mycelium. Previous experiments have shown that fungal lipid synthesized from carbohydrate in the root is one form of exported carbon. In this study, an analysis of the labeling in storage and structural carbohydrates after (13)C(1) glucose was provided to AM roots shows that this is not the only pathway for the flow of carbon from the intraradical to the extraradical mycelium (ERM). Labeling patterns in glycogen, chitin, and trehalose during the development of the symbiosis are consistent with a significant flux of exported glycogen. The identification, among expressed genes, of putative sequences for glycogen synthase, glycogen branching enzyme, chitin synthase, and for the first enzyme in chitin synthesis (glutamine fructose-6-phosphate aminotransferase) is reported. The results of quantifying glycogen synthase gene expression within mycorrhizal roots, germinating spores, and ERM are consistent with labeling observations using (13)C-labeled acetate and glycerol, both of which indicate that glycogen is synthesized by the fungus in germinating spores and during symbiosis. Implications of the labeling analyses and gene sequences for the regulation of carbohydrate metabolism are discussed, and a 4-fold role for glycogen in the AM symbiosis is proposed: sequestration of hexose taken from the host, long-term storage in spores, translocation from intraradical mycelium to ERM, and buffering of intracellular hexose levels throughout the life cycle.
Figures



References
-
- Bago B, Azcón-Aguilar C, Goulet A, Piché Y. Branched absorbing structures (BAS): a feature of the extraradical mycelium of symbiotic arbuscular mycorrhizal fungi. New Phytol. 1998;139:375–388.
-
- Bécard G, Doner LW, Rolin DB, Douds DD, Pfeffer PE. Identification and quantification of trehalose in vesicular-arbuscular mycorrhizal fungi by in vivo 13C NMR and HPLC analyses. New Phytol. 1991;118:547–552.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

