An FeIV=O complex of a tetradentate tripodal nonheme ligand
- PMID: 12644707
- PMCID: PMC152979
- DOI: 10.1073/pnas.0636830100
An FeIV=O complex of a tetradentate tripodal nonheme ligand
Abstract
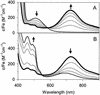
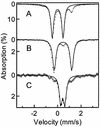
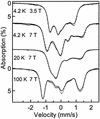
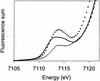
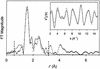
The reaction of [Fe(II)(tris(2-pyridylmethyl)amine, TPA)(NCCH(3))(2)](2+) with 1 equiv. peracetic acid in CH(3)CN at -40 degrees C results in the nearly quantitative formation of a pale green intermediate with lambda(max) at 724 nm ( epsilon approximately 300 M(-1).cm(-1)) formulated as [Fe(IV)(O)(TPA)](2+) by a combination of spectroscopic techniques. Its electrospray mass spectrum shows a prominent feature at mz 461, corresponding to the [Fe(IV)(O)(TPA)(ClO(4))](+) ion. The Mössbauer spectra recorded in zero field reveal a doublet with DeltaE(Q) = 0.92(2) mms and delta = 0.01(2) mms; analysis of spectra obtained in strong magnetic fields yields parameters characteristic of S = 1 Fe(IV)O complexes. The presence of an Fe(IV)O unit is also indicated in its Fe K-edge x-ray absorption spectrum by an intense 1-s --> 3-d transition and the requirement for an ON scatterer at 1.67 A to fit the extended x-ray absorption fine structure region. The [Fe(IV)(O)(TPA)](2+) intermediate is stable at -40 degrees C for several days but decays quantitatively on warming to [Fe(2)(mu-O)(mu-OAc)(TPA)(2)](3+). Addition of thioanisole or cyclooctene at -40 degrees C results in the formation of thioanisole oxide (100% yield) or cyclooctene oxide (30% yield), respectively; thus [Fe(IV)(O)(TPA)](2+) is an effective oxygen-atom transfer agent. It is proposed that the Fe(IV)O species derives from OO bond heterolysis of an unobserved Fe(II)(TPA)-acyl peroxide complex. The characterization of [Fe(IV)(O)(TPA)](2+) as having a reactive terminal Fe(IV)O unit in a nonheme ligand environment lends credence to the proposed participation of analogous species in the oxygen activation mechanisms of many mononuclear nonheme iron enzymes.
Figures

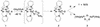
References
-
- Sono M., Roach, M. P., Coulter, E. D. & Dawson, J. H. (1996) Chem. Rev. 96, 2841-2887. - PubMed
-
- Schlichting I., Berendzen, J., Chu, K., Stock, A. M., Maves, S. A., Benson, D. E., Sweet, R. M., Ringe, D., Petsko, G. A. & Sligar, S. G. (2000) Science 287, 1615-1622. - PubMed
-
- Kellner D. G., Hung, S.-C., Weiss, K. E. & Sligar, S. G. (2002) J. Biol. Chem. 277, 9641-9644. - PubMed
-
- Groves J. T. & Han, Y.-Z. (1995) in Cytochrome P450: Structure, Mechanism, and Biochemistry, ed. Ortiz de Montellano, P. R. (Plenum, New York), pp. 3–48.
-
- Watanabe Y. & Fujii, H. (2000) Struct. Bonding 97, 61-89.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

