Phosphatidylinositol 4-kinase type IIalpha is responsible for the phosphatidylinositol 4-kinase activity associated with synaptic vesicles
- PMID: 12646710
- PMCID: PMC153036
- DOI: 10.1073/pnas.0230488100
Phosphatidylinositol 4-kinase type IIalpha is responsible for the phosphatidylinositol 4-kinase activity associated with synaptic vesicles
Abstract
Phosphorylation of inositol phospholipids plays a key role in cellular regulation via the generation of intracellular second messengers. In addition, it represents a mechanism to regulate interactions of the lipid bilayer with proteins and protein scaffolds involved in vesicle budding, cytoskeletal organization, and signaling. Generation of phosphatidylinositol 4-phosphate [PI(4)P] from phosphatidylinositol (PI) is an important step in this metabolic pathway because PI(4)P is a precursor of other important phosphoinositides and has protein binding properties of its own. We report here that a PI 4-kinase (PI4K) activity previously reported on synaptic vesicles is accounted for by the alpha isoform of the recently characterized type II PI4K (PI4KII) family. PI4KIIalpha, which also accounts for the bulk of PI4K activity in brain extracts, is concentrated at synapses and in the region of the Golgi complex in neuronal perikarya. Our results provide new evidence for the occurrence of a cycle of phosphoinositide synthesis and hydrolysis nested within the exo-endocytic cycle of synaptic vesicles and point to PI4KIIalpha as a critical player in this cycle.
Figures





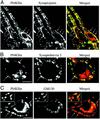
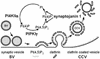
Similar articles
-
A plasma membrane pool of phosphatidylinositol 4-phosphate is generated by phosphatidylinositol 4-kinase type-III alpha: studies with the PH domains of the oxysterol binding protein and FAPP1.Mol Biol Cell. 2005 Mar;16(3):1282-95. doi: 10.1091/mbc.e04-07-0578. Epub 2005 Jan 5. Mol Biol Cell. 2005. PMID: 15635101 Free PMC article.
-
The crystal structure of the phosphatidylinositol 4-kinase IIα.EMBO Rep. 2014 Oct;15(10):1085-92. doi: 10.15252/embr.201438841. Epub 2014 Aug 28. EMBO Rep. 2014. PMID: 25168678 Free PMC article.
-
The WASH complex, an endosomal Arp2/3 activator, interacts with the Hermansky-Pudlak syndrome complex BLOC-1 and its cargo phosphatidylinositol-4-kinase type IIα.Mol Biol Cell. 2013 Jul;24(14):2269-84. doi: 10.1091/mbc.E13-02-0088. Epub 2013 May 15. Mol Biol Cell. 2013. PMID: 23676666 Free PMC article.
-
Inositol phospholipid metabolism in Arabidopsis. Characterized and putative isoforms of inositol phospholipid kinase and phosphoinositide-specific phospholipase C.Plant Physiol. 2002 Sep;130(1):22-46. doi: 10.1104/pp.004770. Plant Physiol. 2002. PMID: 12226484 Free PMC article. Review.
-
Synthesis and function of membrane phosphoinositides in budding yeast, Saccharomyces cerevisiae.Biochim Biophys Acta. 2007 Mar;1771(3):353-404. doi: 10.1016/j.bbalip.2007.01.015. Epub 2007 Feb 6. Biochim Biophys Acta. 2007. PMID: 17382260 Free PMC article. Review.
Cited by
-
Modulation of lipid kinase PI4KIIα activity and lipid raft association of presenilin 1 underlies γ-secretase inhibition by ginsenoside (20S)-Rg3.J Biol Chem. 2013 Jul 19;288(29):20868-20882. doi: 10.1074/jbc.M112.445734. Epub 2013 May 30. J Biol Chem. 2013. PMID: 23723072 Free PMC article.
-
PI4KIIα regulates insulin secretion and glucose homeostasis via a PKD-dependent pathway.Biophys Rep. 2018;4(1):25-38. doi: 10.1007/s41048-018-0049-z. Epub 2018 Mar 7. Biophys Rep. 2018. PMID: 29577067 Free PMC article.
-
PtdIns4KIIα generates endosomal PtdIns(4)P and is required for receptor sorting at early endosomes.Mol Biol Cell. 2016 Mar 15;27(6):990-1001. doi: 10.1091/mbc.E15-08-0564. Epub 2016 Jan 28. Mol Biol Cell. 2016. PMID: 26823017 Free PMC article.
-
Phosphatidylinositol 4-kinases and PI4P metabolism in the nervous system: roles in psychiatric and neurological diseases.Mol Neurobiol. 2013 Feb;47(1):361-72. doi: 10.1007/s12035-012-8358-6. Epub 2012 Oct 10. Mol Neurobiol. 2013. PMID: 23054682 Review.
-
A WASp-binding type II phosphatidylinositol 4-kinase required for actin polymerization-driven endosome motility.J Cell Biol. 2005 Oct 10;171(1):133-42. doi: 10.1083/jcb.200501086. J Cell Biol. 2005. PMID: 16216926 Free PMC article.
References
-
- De Camilli P, Slepnev V I, Shupliakov O, Brodin L. In: Synapses. Cowan W M, Sudhof T C, Stevens C F, editors. Baltimore: Johns Hopkins Univ. Press; 2001. pp. 217–274.
-
- Springer S, Spang A, Schekman R. Cell. 1999;97:145–148. - PubMed
-
- Rothman J E. Nature. 1994;372:55–63. - PubMed
-
- Cremona O, De Camilli P. J Cell Sci. 2001;114:1041–1052. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

