Signaling and regulation of G protein-coupled receptors in airway smooth muscle
- PMID: 12648290
- PMCID: PMC152647
Signaling and regulation of G protein-coupled receptors in airway smooth muscle
Abstract
Signaling through G protein-coupled receptors (GPCRs) mediates numerous airway smooth muscle (ASM) functions including contraction, growth, and "synthetic" functions that orchestrate airway inflammation and promote remodeling of airway architecture. In this review we provide a comprehensive overview of the GPCRs that have been identified in ASM cells, and discuss the extent to which signaling via these GPCRs has been characterized and linked to distinct ASM functions. In addition, we examine the role of GPCR signaling and its regulation in asthma and asthma treatment, and suggest an integrative model whereby an imbalance of GPCR-derived signals in ASM cells contributes to the asthmatic state.
Figures


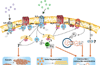

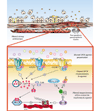
References
-
- Penn RB, Pronin AP, Benovic JL. Regulation of G protein-coupled receptor kinases. Trends Cardiovasc Med. 2000;10:81–89. - PubMed
-
- Barnes PJ. Pharmacology of airway smooth muscle. Am J Respir Crit Care Med. 1998;158(Suppl):S123–S132. - PubMed
-
- Hall IP. Second messengers, ion channels and pharmacology of airway smooth muscle. Eur Respir J. 2000;15:1120–1127. - PubMed
-
- Douglas JS. Receptors on target cells. Receptors on airway smooth muscle. Am Rev Respir Dis. 1990;141(Suppl):S123–S126. - PubMed
-
- Tschumperlin DJ, Drazen JM. Mechanical stimuli to airway remodeling. Am J Respir Crit Care Med. 2001;164(Suppl):S90–S94. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
