Missense mutations in the homeodomain of HOXD13 are associated with brachydactyly types D and E
- PMID: 12649808
- PMCID: PMC1180360
- DOI: 10.1086/374721
Missense mutations in the homeodomain of HOXD13 are associated with brachydactyly types D and E
Abstract
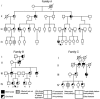
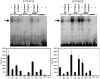
HOXD13, the most 5' gene of the HOXD cluster, encodes a homeodomain transcription factor with important functions in limb patterning and growth. Heterozygous mutations of human HOXD13, encoding polyalanine expansions or frameshifts, are believed to act by dominant negative or haploinsufficiency mechanisms and are predominantly associated with synpolydactyly phenotypes. Here, we describe two mutations of HOXD13 (923C-->G encoding Ser308Cys and 940A-->C encoding Ile314Leu) that cause missense substitutions within the homeodomain. Both are associated with distinctive limb phenotypes in which brachydactyly of specific metacarpals, metatarsals, and phalangeal bones is the most constant feature, exhibiting overlap with brachydactyly types D and E. We investigated the binding of synthetic mutant proteins to double-stranded DNA targets in vitro. No consistent differences were found for the Ser308Cys mutation compared with the wild type, but the Ile314Leu mutation (which resides at the 47th position of the homeodomain) exhibited increased affinity for a target containing the core recognition sequence 5'-TTAC-3' but decreased affinity for a 5'-TTAT-3' target. Molecular modeling of the Ile314Leu mutation indicates that this mixed gain and loss of affinity may be accounted for by the relative positions of methyl groups in the amino acid side chain and target base.
Figures
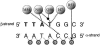




References
Electronic-Database Information
-
- Ensembl, http://www.ensembl.org/Homo_sapiens/mapview?chr=2 (for physical map of 2q31 region)
-
- GenBank, http://www.ncbi.nlm.nih.gov/GenBank/ (for HOXD13 [accession numbers. AF005219, AF005220, AC009336, and NM_000523])
-
- Homeodomain Resource, http://research.nhgri.nih.gov/homeodomain/ (for homeodomain sequences and DNA binding sites)
-
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/(for SPD, HFUS, BDD, and BDE)
-
- Protein Data Bank, http://www.pdb.org/ (for Antp [ID: 9ANT] and Ubx [ID: 1B8I] homeodomain-DNA structures)
References
-
- Albrecht AN, Schwabe GC, Stricker S, Böddrich A, Wanker EE, Mundlos S (2002) The synpolydactyly homolog (spdh) mutation in the mouse: a defect in patterning and growth of limb cartilage elements. Mech Dev 112:53–67 - PubMed
-
- Bell J (1951) On brachydactyly and symphalangism. In: The treasury of human inheritance. Vol 5. Cambridge University Press, Cambridge, pp 1–31
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

