RAGE drives the development of glomerulosclerosis and implicates podocyte activation in the pathogenesis of diabetic nephropathy
- PMID: 12651605
- PMCID: PMC1851245
- DOI: 10.1016/S0002-9440(10)63909-0
RAGE drives the development of glomerulosclerosis and implicates podocyte activation in the pathogenesis of diabetic nephropathy
Abstract
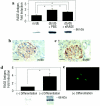
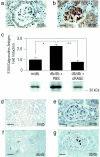
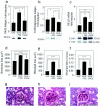
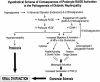
Diabetic nephropathy ensues from events involving earliest changes in the glomeruli and podocytes, followed by accumulation of extracellular matrix in the mesangium. Postulated mechanisms include roles for vascular endothelial growth factor (VEGF), produced by podocytes and contributing to enhanced excretion of urinary albumin and recruitment/activation of inflammatory cells, and transforming growth factor-beta (TGF-beta), elicited largely from mesangial cells and driving production of extracellular matrix. RAGE, a receptor for advanced glycation endproducts (AGEs) and S100/calgranulins, displays enhanced expression in podocytes of genetically diabetic db/db mice by age 13 weeks. RAGE-bearing podocytes express high levels of VEGF by this time, in parallel with enhanced recruitment of mononuclear phagocytes to the glomeruli; events prevented by blockade of RAGE. By age 27 weeks, soluble RAGE-treated db/db mice displayed diminished albuminuria and glomerulosclerosis, and improved renal function. Diabetic homozygous RAGE null mice failed to develop significantly increased mesangial matrix expansion or thickening of the glomerular basement membrane. We propose that activation of RAGE contributes to expression of VEGF and enhanced attraction/activation of inflammatory cells in the diabetic glomerulus, thereby setting the stage for mesangial activation and TGF-beta production; processes which converge to cause albuminuria and glomerulosclerosis.
Figures




References
-
- Ritz E, Rychilik I, Locatelli F, Halimi S: End-stage renal failure in type 2 diabetes: a medical catastrophe of worldwide dimension. Am J Kidney Dis 1999, 34:795-808 - PubMed
-
- Koch M, Kutkuhn B, Grabensee B, Ritz E: Apolipoprotein A, fibrinogen, age, and history of stroke are predictors of death in dialyzed diabetic subjects: prospective study of 412 subjects. Nephrol Dial Transplant 1997, 12:2603-2611 - PubMed
-
- Border WA, Noble NA: Transforming growth factor β in tissue fibrosis. N Engl J Med 1994, 331:1286-1292 - PubMed
-
- Wolf G, Hamann A, Han DC, Helmchen U, Thaiss F, Ziyadeh FN, Stahl RA: Leptin stimulates proliferation and TGF-β expression in renal glomerular endothelial cells: potential role in glomerulosclerosis. Kidney Int 1999, 56:860-872 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

