Comparative efficacy of terbutaline sulphate delivered by Turbuhaler dry powder inhaler or pressurised metered dose inhaler with Nebuhaler spacer in children during an acute asthmatic episode
- PMID: 12651757
- PMCID: PMC1719510
- DOI: 10.1136/adc.88.4.319
Comparative efficacy of terbutaline sulphate delivered by Turbuhaler dry powder inhaler or pressurised metered dose inhaler with Nebuhaler spacer in children during an acute asthmatic episode
Abstract
Aims: To compare the efficacy of terbutaline sulphate delivered via Turbuhaler with a pressurised metered dose inhaler (pMDI) connected to Nebuhaler spacer in a population of asthmatic children presenting to emergency departments because of an acute episode of asthma.
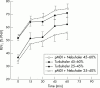
Methods: Randomised double blind, double dummy, parallel study of acute asthma in the emergency department. A total of 112 children (6-16 years), who had a diagnosis of asthma, a baseline FEV1 of 25-60% of predicted normal value (PNV), and the ability to perform spirometry were studied. Patients received two doses of 0.5 mg/10 kg (maximum 2.0 mg) of terbutaline sulphate at time 0 minutes and time 30 minutes. The two groups were also stratified into subgroups based on FEV1: 25-45% and 45.1-60% PNV. FEV1 before treatment and at two 15-minute intervals after each treatment was the main outcome measure. PIF, PEF, heart rate, SpO2, and tremor were also measured at these times.
Results: Both the Turbuhaler and pMDI+Nebuhaler groups showed significant increases from baseline to final value in their FEV1 results, 49% and 50% change from baseline to t = 60 min, respectively (p < 0.001) using last value carried forward. No significant difference was found between the two groups for these results. Subanalysis of the stratified groups revealed similar results. In addition, no significant difference was found in the group and subgroup comparisons for heart rate, SpO2, and tremor.
Conclusion: Results show that Turbuhaler and pMDI+Nebuhaler are similar in terms of benefit and side effects in the treatment of acute moderate to severe asthma attacks in this study population.
Figures