Human and mouse TPIT gene mutations cause early onset pituitary ACTH deficiency
- PMID: 12651888
- PMCID: PMC196015
- DOI: 10.1101/gad.1065603
Human and mouse TPIT gene mutations cause early onset pituitary ACTH deficiency
Abstract
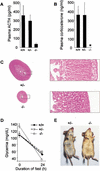
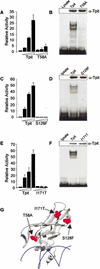
Tpit is a highly cell-restricted transcription factor that is required for expression of the pro-opiomelanocortin (POMC) gene and for terminal differentiation of the pituitary corticotroph lineage. Its exclusive expression in pituitary POMC-expressing cells has suggested that its mutation may cause isolated deficiency of pituitary adrenocorticotropin (ACTH). We now show that Tpit-deficient mice constitute a model of isolated ACTH deficiency (IAD) that is very similar to human IAD patients carrying TPIT gene mutations. Through genetic analysis of a panel of IAD patients, we show that TPIT gene mutations are associated at high frequency with early onset IAD, but not with juvenile forms of this deficiency. We identified seven different TPIT mutations, including nonsense, missense, point deletion, and a genomic deletion. This work defines congenital early onset IAD as a relatively homogeneous clinical entity caused by recessive transmission of loss-of-function mutations in the TPIT gene.
Figures

Comment in
-
Mouse knockout solves endocrine puzzle and promotes new pituitary lineage model.Genes Dev. 2003 Mar 15;17(6):677-82. doi: 10.1101/gad.1085903. Genes Dev. 2003. PMID: 12651886 Review. No abstract available.
References
-
- Argente J, Perez-Jurado L, Sotos J. Molecular bases of pathological growth. J Endocr Genet. 2000;1:179–210.
-
- Bertagna X. Proopiomelanocortin-derived peptides. Endocrinol Metab Clin North Am. 1994;23:467–485. - PubMed
-
- Chowdhary BP, Gustavsson I, Wikberg JE, Chhajlani V. Localization of the human melanocortin-5 receptor gene (MC5R) to chromosome band 18p11.2 by fluorescence in situ hybridization. Cyto Genet Cell Genet. 1995;68:79–81. - PubMed
-
- Cohen LE, Radovick S. Molecular basis of combined pituitary hormone deficiencies. Endocr Rev. 2002;23:431–442. - PubMed
-
- Dattani MT, Martinez-Barbera JP, Thomas PQ, Brickman JM, Gupta R, Martensson IL, Toresson H, Fox M, Wales JK, Hindmarsh PC, et al. Mutations in the homeobox gene HESX1/Hesx1 associated with septo-optic dysplasia in human and mouse. Nat Genet. 1998;19:125–133. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
