Tpit determines alternate fates during pituitary cell differentiation
- PMID: 12651892
- PMCID: PMC196016
- DOI: 10.1101/gad.1065703
Tpit determines alternate fates during pituitary cell differentiation
Abstract
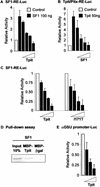
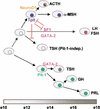
The T-box transcription factor Tpit was identified as a cell-specific factor for expression of the pituitary proopiomelanocortin (POMC) gene. Expression of this factor is exclusively restricted to the pituitary POMC-expressing lineages, the corticotrophs and melanotrophs. We have now determined the role of this factor in pituitary cell differentiation. Tpit is a positive regulator for late POMC cell differentiation and POMC expression, but it is not essential for lineage commitment. The pituitary intermediate lobe normally contains only Tpit-expressing melanotrophs. Inactivation of the Tpit gene results in almost complete loss of POMC-expressing cells in this tissue, which now has a large number of gonadotrophs and a few clusters of Pit-1-independent thyrotrophs. The role of Tpit as a negative regulator of gonadotroph differentiation was confirmed in transgenic gain-of-function experiments. One mechanism to account for the negative role of Tpit in differentiation may be trans-repression between Tpit and the gonadotroph-restricted factor SF1. These data suggest that antagonism between Tpit and SF1 may play a role in establishment of POMC and gonadotroph lineages and that these lineages may arise from common precursors.
Figures




References
-
- Bodner M, Castrillo JL, Theill LE, Deerinck T, Ellisman M, Karin M. The pituitary-specific transcription factor GHF-1 is a homeobox-containing protein. Cell. 1988;55:505–518. - PubMed
-
- Daikoku S, Chikamori M, Adachi T, Maki Y. Effect of the basal diencephalon on the development of Rathke's pouch in rats: A study in combined organ cultures. Dev Biol. 1982;90:198–202. - PubMed
-
- Dasen JS, O'Connell SM, Flynn SE, Treier M, Gleiberman AS, Szeto DP, Hooshmand F, Aggarwal AK, Rosenfeld MG. Reciprocal interactions of Pit1 and GATA2 mediate signaling gradient-induced determination of pituitary cell types. Cell. 1999;97:587–598. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
