GTP-induced tetrodotoxin-resistant Na+ current regulates excitability in mouse and rat small diameter sensory neurones
- PMID: 12651922
- PMCID: PMC2342858
- DOI: 10.1113/jphysiol.2003.039131
GTP-induced tetrodotoxin-resistant Na+ current regulates excitability in mouse and rat small diameter sensory neurones
Abstract
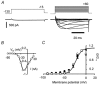
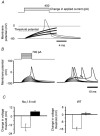
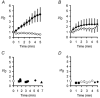
Peripheral pain thresholds are regulated by the actions of inflammatory mediators. Some act through G-protein-coupled receptors on voltage-gated sodium channels. We have found that a low-threshold, persistent tetrodotoxin-resistant Na+ current, attributed to NaV1.9, is upregulated by GTP and its non-hydrolysable analogue GTP-gamma-S, but not by GDP. Inclusion of GTP-gamma-S (500 microM) in the internal solution led to an increase in maximal current amplitude of > 300 % within 5 min. In current clamp, upregulation of persistent current was associated with a more negative threshold for action potential induction (by 15-16 mV) assessed from a holding potential of -90 mV. This was not seen in neurones without the low-threshold current or with internal GDP (P < 0.001). In addition, persistent current upregulation depolarized neurones. At -60 mV, internal GTP-gamma-S led to the generation of spontaneous activity in initially silent neurones only when persistent current was upregulated. These findings suggest that regulation of the persistent current has important consequences for nociceptor excitability.
Figures



References
-
- Akopian AN, Okuse K, Souslova V, England S, Ogata N, Wood JN. Trans-splicing of a voltage-gated sodium channel is regulated by nerve growth factor. FEBS Lett. 1999a;445:177–182. - PubMed
-
- Akopian AN, Souslova V, England S, Okuse K, Ogata N, Ure J, Smith A, Kerr BJ, McMahon SB, Boyce S, Hill R, Stanfa LC, Dickenson AH, Wood JN. The tetrodotoxin-resistant sodium channel SNS has a specialized function in pain pathways. Nat Neurosci. 1999b;2:541–548. - PubMed
-
- Amaya F, Decosterd I, Samad TA, Plumpton C, Tate S, Mannion RJ, Costigan M, Woolf CJ. Diversity of expression of the sensory neuron-specific TTX-resistant voltage-gated sodium ion channels SNS and SNS2. Mol Cell Neurosci. 2000;15:331–342. - PubMed
-
- Baker MD, Bostock H. Low-threshold, persistent sodium current in rat large dorsal root ganglion neurons in culture. J Neurophys. 1997;77:1503–1513. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

