Acute regulation of the SLC26A3 congenital chloride diarrhoea anion exchanger (DRA) expressed in Xenopus oocytes
- PMID: 12651923
- PMCID: PMC2342915
- DOI: 10.1113/jphysiol.2003.039818
Acute regulation of the SLC26A3 congenital chloride diarrhoea anion exchanger (DRA) expressed in Xenopus oocytes
Abstract
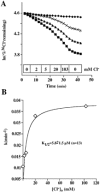
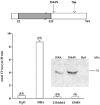
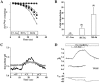
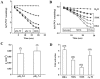
Mutations in the human SLC26A3 gene, also known as down-regulated in adenoma (hDRA), cause autosomal recessive congenital chloride-losing diarrhoea (CLD). hDRA expressed in Xenopus oocytes mediated bidirectional Cl--Cl- and Cl--HCO3- exchange. In contrast, transport of oxalate was low, and transport of sulfate and of butyrate was undetectable. Two CLD missense disease mutants of hDRA were nonfunctional in oocytes. Truncation of up to 44 C-terminal amino acids from the putatively cytoplasmic C-terminal hydrophilic domain left transport function unimpaired, but deletion of the adjacent STAS (sulfate transporter anti-sigma factor antagonist) domain abolished function. hDRA-mediated Cl- transport was insensitive to changing extracellular pH, but was inhibited by intracellular acidification and activated by NH4+ at acidifying concentrations. These regulatory responses did not require the presence of either hDRA's N-terminal cytoplasmic tail or its 44 C-terminal amino acids, but they did require more proximate residues of the C-terminal cytoplasmic domain. Although only weakly sensitive to inhibition by stilbenes, hDRA was inhibited with two orders of magnitude greater potency by the anti-inflammatory drugs niflumate and tenidap. cAMP-insensitive Cl--HCO3- exchange mediated by hDRA gained modest cAMP sensitivity when co-expressed with cystic fibrosis transmembrane conductance regulator (CFTR). Despite the absence of hDRA transcripts in human cell lines derived from CFTR patients, DRA mRNA was present at wild-type levels in proximal colon and nearly so in the distal ileum of CFTR(-/-) mice. Thus, pharmacological modulation of DRA might be a useful adjunct treatment of cystic fibrosis.
Figures


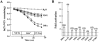
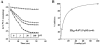


References
-
- Aichbichler BW, Zerr CH, Santa Ana CA, Porter JL, Fordtran JS. Proton-pump inhibition of gastric chloride secretion in congenital chloridorrhea. N Engl J Med. 1997;336:106–109. - PubMed
-
- Alper SL. Genetic diseases of acid-base transport. Annu Rev Physiol. 2002;64:899–923. - PubMed
-
- Antalis TM, Reeder JA, Gotley DC, Byeon MK, Walsh MD, Henderson KW, Papas TS, Schweinfest CA. Down-regulation of the Down-Regulated in Adenoma (DRA) gene correlates with colon tumor progression. Clin Cancer Res. 1998;4:1857–1863. - PubMed
-
- Aravind L, Koonin EV. The STAS domain-a link between anion transporters and antisigma-factor antagonists. Curr Biol. 2000;10:R53, R55. - PubMed
-
- Bennekou P, Pedersen O, Moller A, Christophersen P. Volume control in sickle cells is facilitated by the novel anion conductance inhibitor NS1652. Blood. 2000;5:1842–1848. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

