Genetic characterization of glucose transporter function in Leishmania mexicana
- PMID: 12651954
- PMCID: PMC153020
- DOI: 10.1073/pnas.0630165100
Genetic characterization of glucose transporter function in Leishmania mexicana
Erratum in
- Proc Natl Acad Sci U S A. 2003 May 13;100(10):6287
Abstract
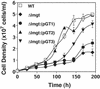
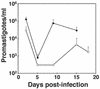
Both insect and mammalian life cycle stages of Leishmania mexicana take up glucose and express all three isoforms encoded by the LmGT glucose transporter gene family. To evaluate glucose transporter function in intact parasites, a null mutant line has been created by targeted disruption of the LmGT locus that encompasses the LmGT1, LmGT2, and LmGT3 genes. This deltalmgt null mutant exhibited no detectable glucose transport activity. The growth rate of the deltalmgt knockout in the promastigote stage was reduced to a rate comparable with that of WT cells grown in the absence of glucose. deltalmgt cells also exhibited dramatically reduced infectivity to macrophages, demonstrating that expression of LmGT isoforms is essential for viability of amastigotes. Furthermore, WT L. mexicana were not able to grow as axenic culture form amastigotes if glucose was withdrawn from the medium, implying that glucose is an essential nutrient in this life cycle stage. Expression of either LmGT2 or LmGT3, but not of LmGT1, in deltalmgt null mutants significantly restored growth as promastigotes, but only LmGT3 expression substantially rescued amastigote growth in macrophages. Subcellular localization of the three isoforms was investigated in deltalmgt cells expressing individual LmGT isoforms. Using anti-LmGT antiserum and GFP-tagged LmGT fusion proteins, LmGT2 and LmGT3 were localized to the cell body, whereas LmGT1 was localized specifically to the flagellum. These results establish that each glucose transporter isoform has distinct biological functions in the parasite.
Figures
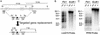

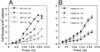


References
-
- Anez N, Luga A, Loaiza A, Nieves D, Orozco J. Med Vet Entomol. 1994;8:38–42. - PubMed
-
- Sacks D L, Perkins P V. Science. 1984;223:1417–1419. - PubMed
-
- Schlein Y. Parasitol Today. 1986;2:175–177. - PubMed
-
- Tang Y, Ward R D. Med Vet Entomol. 1988;12:13–19. - PubMed
-
- Richards A G. Acta Trop. 1975;32:83–95. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

