Tuning the free-energy landscape of a WW domain by temperature, mutation, and truncation
- PMID: 12651955
- PMCID: PMC153028
- DOI: 10.1073/pnas.0538054100
Tuning the free-energy landscape of a WW domain by temperature, mutation, and truncation
Abstract
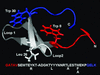
The equilibrium unfolding of the Formin binding protein 28 (FBP) WW domain, a stable three-stranded beta-sheet protein, can be described as reversible apparent two-state folding. Kinetics studied by laser temperature jump reveal a third state at temperatures below the midpoint of unfolding. The FBP free-energy surface can be tuned between three-state and two-state kinetics by changing the temperature, by truncation of the C terminus, or by selected point mutations. FBP WW domain is the smallest three-state folder studied to date and the only one that can be freely tuned between three-state and apparent two-state folding by several methods (temperature, truncation, and mutation). Its small size (28-37 residues), the availability of a quantitative reaction coordinate (phi(T)), the fast folding time scale (10s of micros), and the tunability of the folding routes by small temperature or sequence changes make this system the ideal prototype for studying more subtle features of the folding free-energy landscape by simulations or analytical theory.
Figures
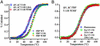

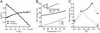

Comment in
-
Meeting halfway on the bridge between protein folding theory and experiment.Proc Natl Acad Sci U S A. 2003 Apr 1;100(7):3555-6. doi: 10.1073/pnas.0830965100. Epub 2003 Mar 25. Proc Natl Acad Sci U S A. 2003. PMID: 12657736 Free PMC article. No abstract available.
References
-
- Sudol M. Prog Biophys Mol Biol. 1996;65:113–132. - PubMed
-
- Sudol M, Hunter T. Cell. 2000;103:1001–1004. - PubMed
-
- Jäger M, Nguyen H, Crane J, Kelly J, Gruebele M. J Mol Biol. 2001;311:373–393. - PubMed
-
- Kaul R, Angeles A, Jäger M, Powers E T, Kelly J W. J Am Chem Soc. 2001;123:5206–5212. - PubMed
-
- Toepert F, Pires J R, Landgraf C, Oschkinat H, Schneider-Mergener J. Angew Chem Int Ed Engl. 2001;40:897–900. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

