Lipid interaction differentiates the constitutive and stress-induced heat shock proteins Hsc70 and Hsp70
- PMID: 12653477
- PMCID: PMC514832
- DOI: 10.1379/1466-1268(2002)007<0330:lidtca>2.0.co;2
Lipid interaction differentiates the constitutive and stress-induced heat shock proteins Hsc70 and Hsp70
Abstract
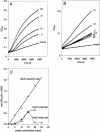
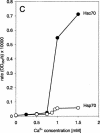
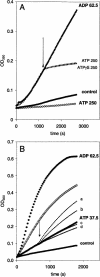
Heat shock proteins play a major role in the process of protein folding, and they have been termed molecular chaperones. Two members of the Hsp70 family, Hsc70 and Hsp70, have a high degree of sequence homology. But they differ in their expression pattern. Hsc70 is constitutively expressed, whereas Hsp70 is stress inducible. These 2 proteins are localized in the cytosol and the nucleus. In addition, they have also been observed in close proximity to cellular membranes. We have recently reported that Hsc70 is capable of interacting with a lipid bilayer forming ion-conductance channels. In the present study, we found that both Hsc70 and Hsp70 interact with lipids and can be differentiated by their characteristic induction of liposome aggregation. These proteins promote the aggregation of phosphatidylserine liposomes in a time- and protein concentration-dependent manner. Although both proteins are active in this process, the level and kinetics of aggregation are different between them. Calcium ions enhance Hsc70 and Hsp70 liposome aggregation, but the effect is more dramatic for Hsc70 than for Hsp70. Addition of adenosine triphosphate blocks liposome aggregation induced by both proteins. Adenosine diphosphate (ADP) also blocks Hsp70-mediated liposome aggregation. Micromolar concentrations of ADP enhance Hsc70-induced liposome aggregation, whereas at millimolar concentrations the nucleotide has an inhibitory effect. These results confirm those of previous studies indicating that the Hsp70 family can interact with lipids directly. It is possible that the interaction of Hsp70s with lipids may play a role in the folding of membrane proteins and the translocation of polypeptides across membranes.
Figures



References
-
- Agashe VR, Hartl FU. Roles of molecular chaperones in cytoplasmic protein folding. Semin Cell Dev Biol. 2000;1(1):15–25. - PubMed
-
- Arispe N, De Maio A. ATP and ADP modulate a cation channel formed by Hsc70 in acid phospholipid membranes. J Biol Chem. 2000a;275:30839–30843. - PubMed
-
- Arispe N, De Maio A 2000b Differential liposome aggregation by two members of the heat shock 70 family of proteins. Biophys J. 78(1):36. A.
-
- Arispe N, Doh M, and De Maio A 2001 Extracellular interaction of the heat shock proteins Hsc70 and Hsp70 with PC12 cells leads to a reduction in viability [abstract]. Mol Biol Cell. 12:201. A.
-
- Asea A, Kraeft SK, Kurt-Jones EA, Stevenson MA, Chen LB, Finberg RW, Koo GC, Caldewood SK. Hsp70 stimulates cytokine production through a CD 14-dependent pathway, demonstrating its dual role as a chaperone and cytokine. Nat Med. 2000;6:435–442. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
