Toxoplasma gondii-derived heat shock protein HSP70 functions as a B cell mitogen
- PMID: 12653480
- PMCID: PMC514835
- DOI: 10.1379/1466-1268(2002)007<0357:tgdhsp>2.0.co;2
Toxoplasma gondii-derived heat shock protein HSP70 functions as a B cell mitogen
Abstract
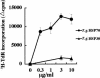
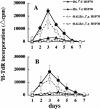
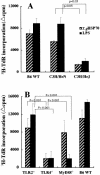
We have investigated the role of Toxoplasma gondii-derived heat shock protein 70 (TgHSP70) as a B cell mitogen by measuring proliferative responses in vitro. TgHSP70 induced prominent proliferative responses in murine B cells derived not only from T gondii-infected but also from uninfected mice. Nude mice responded to TgHSP70; however, severe combined immunodeficiency, RAG1-/- B6, and microMT mice failed to respond. B220+ spleen cells showed marked proliferation after stimulation with TgHSP70, but neither CD4+ nor CD8+ population responded. This unresponsiveness of CD4+ and CD8- T cells to TgHSP70 was antigen presenting cells independent. These data indicate that TgHSP70 induced the proliferation of B cells but not T cells. Polymyxin B, a potent inhibitor of lipopolysaccharide (LPS), did not eliminate TgHSP70-induced proliferation. C3H/HeN mice responded well to TgHSP70 stimulation; however, C3H/HeJ mice carrying a point mutation in the Toll-like receptor (TLR) 4 failed to respond. This indicates that TLR4 is required for TgHSP70-induced B cell activation. The involvement of TLR4 in the TgHSP70-induced proliferative responses of spleen cells was also shown by the use of TLR4-/- mice. But TgHSP70-induced, but not LPS-induced, spleen cell proliferation was observed in MyD88-/- mice, indicating that the MyD88 molecule was involved in LPS-induced proliferation but not in TgHSP70-induced proliferation.
Figures






References
-
- Akashi S, Shimazu R, Ogata H, Nagai Y, Takeda K, Kimoto M, Miyake K. Cutting edge: cell surface expression and lipopolysaccharide signaling via the toll-like receptor 4-MD-2 complex on mouse peritoneal macrophages. J Immunol. 2000;164:3471–3475. - PubMed
-
- Arnold-Schild D, Hanau D, Spehner D, Schmid C, Rammensee HG, de la Salle H, Schild H. Cutting edge: receptor-mediated endocytosis of heat shock proteins by professional antigen-presenting cells. J Immunol. 1999;162:3757–3760. - PubMed
-
- Asea A, Kraeft SK, and Kurt-Jones EA. et al. 2000 HSP70 stimulates cytokine production through a CD14-dependant pathway, demonstrating its dual role as a chaperone and cytokine. Nat Med. 6:435–442. - PubMed
-
- Basu S, Binder RJ, Suto R, Anderson KM, Srivastava PK. Necrotic but not apoptotic cell death releases heat shock proteins, which deliver a partial maturation signal to dendritic cells and activate the NF-kappa B pathway. Int Immunol. 2000;12:1539–1546. - PubMed
-
- Bonorino C, Nardi NB, Zhang X, Wysocki LJ. Characteristics of the strong antibody response to mycobacterial Hsp70: a primary, T cell-dependent IgG response with no evidence of natural priming or gamma delta T cell involvement. J Immunol. 1998;161:5210–5216. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Medical
Research Materials
