Role of nutrient limitation and stationary-phase existence in Klebsiella pneumoniae biofilm resistance to ampicillin and ciprofloxacin
- PMID: 12654654
- PMCID: PMC152508
- DOI: 10.1128/AAC.47.4.1251-1256.2003
Role of nutrient limitation and stationary-phase existence in Klebsiella pneumoniae biofilm resistance to ampicillin and ciprofloxacin
Abstract
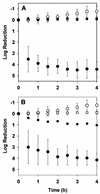
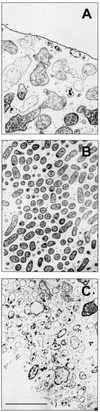
Biofilms formed by Klebsiella pneumoniae resisted killing during prolonged exposure to ampicillin or ciprofloxacin even though these agents have been shown to penetrate bacterial aggregates. Bacteria dispersed from biofilms into medium quickly regained most of their susceptibility. Experiments with free-floating bacteria showed that stationary-phase bacteria were protected from killing by either antibiotic, especially when the test was performed in medium lacking carbon and nitrogen sources. These results suggested that the antibiotic tolerance of biofilm bacteria could be explained by nutrient limitation in the biofilm leading to stationary-phase existence of at least some of the cells in the biofilm. This mechanism was supported by experimental characterization of nutrient availability and growth status in biofilms. The average specific growth rate of bacteria in biofilms was only 0.032 h(-1) compared to the specific growth rate of planktonic bacteria of 0.59 h(-1) measured in the same medium. Glucose did not penetrate all the way through the biofilm, and oxygen was shown to penetrate only into the upper 100 micro m. The specific catalase activity was elevated in biofilm bacteria to a level similar to that of stationary-phase planktonic cells. Transmission electron microscopy revealed that bacteria were affected by ampicillin near the periphery of the biofilm but were not affected in the interior. Taken together, these results indicate that K. pneumoniae in this system experience nutrient limitation locally within the biofilm, leading to zones in which the bacteria enter stationary phase and are growing slowly or not at all. In these inactive regions, bacteria are less susceptible to killing by antibiotics.
Figures




References
-
- Ashby, M. J., J. E. Neale, S. J. Knott, and I. A. Critchley. 1994. Effect of antibiotics on non-growing planktonic cells and biofilms of Escherichia coli. J. Antimicrob. Chemother. 33:443-452. - PubMed
-
- Brown, M. R. W., D. G. Allison, and P. Gilbert. 1988. Resistance of bacterial biofilms to antibiotics: a growth rate-related effect? J. Antimicrob. Chemother. 22:777-783. - PubMed
-
- Costerton, J. W., P. S. Stewart, and E. P. Greenberg. 1999. Bacterial biofilms: a common cause of persistent infections. Science 284:1318-1322. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

