Daptomycin pharmacokinetics and safety following administration of escalating doses once daily to healthy subjects
- PMID: 12654665
- PMCID: PMC152488
- DOI: 10.1128/AAC.47.4.1318-1323.2003
Daptomycin pharmacokinetics and safety following administration of escalating doses once daily to healthy subjects
Abstract
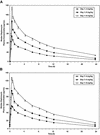
The purpose of this paper is to establish the pharmacokinetics and safety of escalating, once-daily doses of daptomycin, a novel lipopeptide antibiotic active against gram-positive pathogens, including those resistant to methicillin and vancomycin. This phase 1, multiple-dose, double-blind study involved 24 healthy subjects in three dose cohorts (4, 6, and 8 mg/kg of body weight) who were randomized to receive daptomycin or the control at a 3:1 ratio and administered the study medication by a 30-min intravenous infusion every 24 h for 7 to 14 days. Daptomycin pharmacokinetics was assessed by blood and urine sampling. Safety and tolerability were evaluated by monitoring adverse events (AEs) and laboratory parameters. Daptomycin pharmacokinetics was linear through 6 mg/kg, with a slight ( approximately 20%) nonlinearity in the area under the curve and trough concentration at the highest dose studied (8 mg/kg). The pharmacokinetic parameters measured on the median day of the study period, (day 7) were half-life ( approximately 9 h), volume of distribution ( approximately 0.1 liters/kg), systemic clearance ( approximately 8.2 ml/h/kg), and percentage of the drug excreted intact in urine from 0 to 24 h ( approximately 54%). Daptomycin protein binding (mean amount bound, 91.7%) was independent of the drug concentration. No gender effect was observed. All subjects who received daptomycin completed the study. The frequencies and distributions of treatment-emergent AEs were similar for the subjects who received daptomycin and the control subjects. There were no serious AEs and no pattern of dose-related events. The pharmacokinetics of once-daily administration of daptomycin was linear through 6 mg/kg. For all three doses, plasma daptomycin concentrations were consistent and predictable throughout the dosing interval. Daptomycin was well tolerated when it was administered once daily at a dose as high as 8 mg/kg for 14 days.
Figures

References
-
- Byrnes, W. C., P. M. Clarkson, J. S. White, S. S. Hsieh, P. N. Frykman, and R. J. Maughan. 1985. Delayed onset muscle soreness following repeated bouts of downhill running. J. Appl. Physiol. 59:710-715. - PubMed
-
- Cassell, G. H., and J. Mekalanos. 2001. Development of antimicrobial agents in the era of new and reemerging infectious diseases and increasing antibiotic resistance. JAMA 285:601-605. - PubMed
-
- Gibaldi, M., and D. Perrier. 1982. Pharmacokinetics, 2nd ed. Marcel Dekker, New York, N.Y.
-
- Hanberger, H., L. E. Nilsson, R. Maller, and B. Isaksson. 1991. Pharmacodynamics of daptomycin and vancomycin on Enterococcus faecalis and Staphylococcus aureus demonstrated by studies of initial killing and postantibiotic effect and influence of Ca2+ and albumin on these drugs. Antimicrob. Agents Chemother. 35:1710-1716. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

