Phase I safety and pharmacokinetic trials of 1263W94, a novel oral anti-human cytomegalovirus agent, in healthy and human immunodeficiency virus-infected subjects
- PMID: 12654667
- PMCID: PMC152490
- DOI: 10.1128/AAC.47.4.1334-1342.2003
Phase I safety and pharmacokinetic trials of 1263W94, a novel oral anti-human cytomegalovirus agent, in healthy and human immunodeficiency virus-infected subjects
Abstract
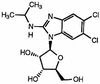
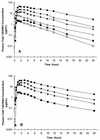
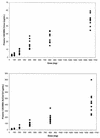
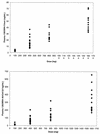
1263W94 [maribavir; 5,6-dichloro-2-(isopropylamino)-1, beta-L-ribofuranosyl-1-H-benzimidazole], a novel benzimidazole compound, has been demonstrated to potently and selectively inhibit human cytomegalovirus replication in vitro and to have favorable safety profiles in animal species. Two phase I trials evaluated the safety and pharmacokinetics of escalating single doses of 1263W94 in 13 healthy subjects (dose, 50 to 1,600 mg) and 17 human immunodeficiency virus (HIV)-infected subjects (dose, 100 to 1,600 mg). No severe safety concerns were observed in the evaluation of adverse events, vital signs, electrocardiograms, and clinical laboratory tests following administration of a single dose of 1263W94. The most frequently reported adverse events in both populations were taste disturbance (80%) and headache (53%). 1263W94 was rapidly absorbed following oral administration, with peak concentrations in plasma (C(max)) occurring 1 to 3 h after dosing. The increases in the C(max) of 1263W94 and the area under the concentration-time curve from time zero to infinity (AUC(0- infinity )) for 1263W94 were dose dependent; C(max) increased slightly less than proportionally to the dose, and AUC(0- infinity ) increased slightly more than proportionally to the dose. 1263W94 was rapidly eliminated, with a mean half-life in plasma of 3 to 5 h; the half-life was independent of the dose level. Less than 2% of the 1263W94 dose administered was eliminated unchanged in urine. The principal metabolite of 1263W94 was 4469W94 (which is derived by N-dealkylation of 1263W94 via CYP3A4), which accounted for 30 to 40% of the dose in urine. Greater than 98% of the 1263W94 in plasma is bound to proteins, and the extent of binding appears to be constant over the dose range of 200 to 1,600 mg. In the trial with HIV-infected subjects, consumption of a high-fat meal decreased the 1263W94 AUC(0- infinity ) and C(max) in plasma by approximately 30%.
Figures
Similar articles
-
Phase I dose escalation trial evaluating the pharmacokinetics, anti-human cytomegalovirus (HCMV) activity, and safety of 1263W94 in human immunodeficiency virus-infected men with asymptomatic HCMV shedding.Antimicrob Agents Chemother. 2002 Sep;46(9):2969-76. doi: 10.1128/AAC.46.9.2969-2976.2002. Antimicrob Agents Chemother. 2002. PMID: 12183255 Free PMC article. Clinical Trial.
-
Maribavir: 1263W94, Benzimidavir, GW 1263, GW 1263W94, VP41263.Drugs R D. 2007;8(3):188-92. doi: 10.2165/00126839-200708030-00006. Drugs R D. 2007. PMID: 17472414 Review.
-
Preclinical and toxicology studies of 1263W94, a potent and selective inhibitor of human cytomegalovirus replication.Antimicrob Agents Chemother. 2002 Aug;46(8):2373-80. doi: 10.1128/AAC.46.8.2373-2380.2002. Antimicrob Agents Chemother. 2002. PMID: 12121907 Free PMC article.
-
Synthesis and evaluation of a series of 2'-deoxy analogues of the antiviral agent 5,6-dichloro-2-isopropylamino-1-(beta-L-ribofuranosyl)-1H-benzimidazole (1263W94).Nucleosides Nucleotides Nucleic Acids. 2000 Jan-Feb;19(1-2):101-23. doi: 10.1080/15257770008032999. Nucleosides Nucleotides Nucleic Acids. 2000. PMID: 10772705
-
Maribavir: a novel antiviral agent with activity against cytomegalovirus.Ann Pharmacother. 2008 Oct;42(10):1447-57. doi: 10.1345/aph.1L065. Epub 2008 Aug 12. Ann Pharmacother. 2008. PMID: 18698013 Review.
Cited by
-
[Refractory or resistant cytomegalovirus infections after hematopoietic stem cell transplantation: diagnosis and management].Zhonghua Xue Ye Xue Za Zhi. 2024 Nov 14;45(11):1058-1064. doi: 10.3760/cma.j.cn121090-20240615-00223. Zhonghua Xue Ye Xue Za Zhi. 2024. PMID: 39746704 Free PMC article. Review. Chinese.
-
Human papillomavirus 16 E7 inactivator of retinoblastoma family proteins complements human cytomegalovirus lacking UL97 protein kinase.Proc Natl Acad Sci U S A. 2009 Sep 29;106(39):16823-8. doi: 10.1073/pnas.0901521106. Epub 2009 Sep 15. Proc Natl Acad Sci U S A. 2009. PMID: 19805380 Free PMC article.
-
Update on human herpesvirus 6 biology, clinical features, and therapy.Clin Microbiol Rev. 2005 Jan;18(1):217-45. doi: 10.1128/CMR.18.1.217-245.2005. Clin Microbiol Rev. 2005. PMID: 15653828 Free PMC article. Review.
-
Maribavir: Mechanism of action, clinical, and translational science.Clin Transl Sci. 2024 Jan;17(1):e13696. doi: 10.1111/cts.13696. Clin Transl Sci. 2024. PMID: 38071422 Free PMC article. Review.
-
Current Perspectives on Letermovir and Maribavir for the Management of Cytomegalovirus Infection in Solid Organ Transplant Recipients.Drug Des Devel Ther. 2024 Sep 6;18:3987-4001. doi: 10.2147/DDDT.S265644. eCollection 2024. Drug Des Devel Ther. 2024. PMID: 39258274 Free PMC article. Review.
References
-
- Biron, K. K., R. J. Harvey, S. C. Chamberlain, S. S. Good, A. A. Smith III, M. G. Davis, C. L. Talarico, W. H. Miller, R. Ferris, R. E. Dornsife, S. C. Stanat, J. C. Drach, L. B. Townsend, and G. W. Koszalka. 2002. Potent and selective inhibition of human cytomegalovirus replication by 1263W94, a benzimidazole l-riboside with a unique mode of action. Antimicrob. Agents Chemother. 46:2365-2372. - PMC - PubMed
-
- Chan, J., S. Chamberlain, K. Biron, M. Davis, R. Harvey, D. Selleseth, R. Dornsife, E. Dark, L. Frick, L. Townsend, J. Drach, and G. Koszalka. 2000. Synthesis and evaluation of a series of 2′-deoxy analogues of the antiviral agent 5,6-dichloro-2-isopropylamino-1-(β-l-ribofuranosyl)-1H-benzimidazole (1263W94). Nucleosides Nucleotides Nucleic Acids 19:101-123. - PubMed
-
- de la Hoz, R. E., G. Stephens, and C Sherlock. 2002. Diagnosis and treatment approaches to CMV infections in adult patients. J. Clin. Virol. 25:S1-S12. - PubMed
-
- Gaytant, M. A., E. A. Steegers, B. A. Semmekrot, H. M. Merkus, and J. M. Galama. 2002. Congenital cytomegalovirus infection: review of the epidemiology and outcome. Obstet. Gynecol. Surv. 57:245-256. - PubMed
-
- Griffiths, P. 2002. The treatment of cytomegalovirus infection. J. Antimicrob. Chemother. 49:243-253. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

