Intranasal immunization with recombinant Lactococcus lactis secreting murine interleukin-12 enhances antigen-specific Th1 cytokine production
- PMID: 12654805
- PMCID: PMC152106
- DOI: 10.1128/IAI.71.4.1887-1896.2003
Intranasal immunization with recombinant Lactococcus lactis secreting murine interleukin-12 enhances antigen-specific Th1 cytokine production
Abstract
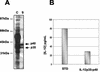
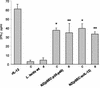
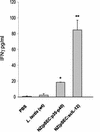
Interleukin-12 (IL-12), a heterodimeric cytokine, plays an important role in cellular immunity to several bacterial, viral, and parasitic infections and has adjuvant activity when it is codelivered with DNA vaccines. IL-12 has also been used with success in cancer immunotherapy treatments. However, systemic IL-12 therapy has been limited by high levels of toxicity. We describe here inducible expression and secretion of IL-12 in the food-grade lactic acid bacterium Lactococcus lactis. IL-12 was expressed as two separate polypeptides (p35-p40) or as a single recombinant polypeptide (scIL-12). The biological activity of IL-12 produced by the recombinant L. lactis strain was confirmed in vitro by its ability to induce gamma interferon (IFN-gamma) production by mouse splenocytes. Local administration of IL-12-producing strains at the intranasal mucosal surface resulted in IFN-gamma production in mice. The activity was greater with the single polypeptide scIL-12. An antigen-specific cellular response (i.e., secretion of Th1 cytokines, IL-2, and IFN-gamma) elicited by a recombinant L. lactis strain displaying a cell wall-anchored human papillomavirus type 16 E7 antigen was dramatically increased by coadministration with an L. lactis strain secreting IL-12 protein. Our data show that IL-12 is produced and secreted in an active form by L. lactis and that the strategy which we describe can be used to enhance an antigen-specific immune response and to stimulate local mucosal immunity.
Figures


Similar articles
-
An inducible surface presentation system improves cellular immunity against human papillomavirus type 16 E7 antigen in mice after nasal administration with recombinant lactococci.J Med Microbiol. 2004 May;53(Pt 5):427-433. doi: 10.1099/jmm.0.05472-0. J Med Microbiol. 2004. PMID: 15096553
-
Influence of the route of immunization and the nature of the bacterial vector on immunogenicity of mucosal vaccines based on lactic acid bacteria.Vaccine. 2007 Sep 4;25(36):6581-8. doi: 10.1016/j.vaccine.2007.06.062. Epub 2007 Jul 23. Vaccine. 2007. PMID: 17675182
-
Protection against human papillomavirus type 16-induced tumors in C57BL/6 mice by mucosal vaccination with Lactococcus lactis NZ9000 expressing E6 oncoprotein.Microb Pathog. 2019 Jan;126:149-156. doi: 10.1016/j.micpath.2018.10.043. Epub 2018 Nov 2. Microb Pathog. 2019. PMID: 30391536
-
Current prophylactic and therapeutic uses of a recombinant Lactococcus lactis strain secreting biologically active interleukin-12.J Mol Microbiol Biotechnol. 2008;14(1-3):80-9. doi: 10.1159/000106086. J Mol Microbiol Biotechnol. 2008. PMID: 17957114 Review.
-
Interleukin 12 and innate molecules for enhanced mucosal immunity.Immunol Res. 1999;20(3):207-17. doi: 10.1007/BF02790404. Immunol Res. 1999. PMID: 10741861 Review.
Cited by
-
Effect of Ampicillin on the kinetics of colonization of Streptococcus pneumoniae and Lactobacillus fermentum in the respiratory tract of mice.Ann Clin Microbiol Antimicrob. 2004 Oct 27;3:23. doi: 10.1186/1476-0711-3-23. Ann Clin Microbiol Antimicrob. 2004. PMID: 15509298 Free PMC article.
-
Display of recombinant proteins at the surface of lactic acid bacteria: strategies and applications.Microb Cell Fact. 2016 May 3;15:70. doi: 10.1186/s12934-016-0468-9. Microb Cell Fact. 2016. PMID: 27142045 Free PMC article. Review.
-
Oral vaccination with novel Lactococcus lactis mucosal live vector-secreting Brucella lumazine synthase (BLS) protein induces humoral and cellular immune protection against Brucella abortus.Arch Microbiol. 2023 Mar 20;205(4):122. doi: 10.1007/s00203-023-03471-6. Arch Microbiol. 2023. PMID: 36939918 Free PMC article.
-
Production of human papillomavirus type 16 L1 virus-like particles by recombinant Lactobacillus casei cells.Appl Environ Microbiol. 2006 Jan;72(1):745-52. doi: 10.1128/AEM.72.1.745-752.2006. Appl Environ Microbiol. 2006. PMID: 16391114 Free PMC article.
-
Analysis of Immune Responses in Mice Orally Immunized with Recombinant pMG36e-SP-TSOL18/Lactococcus lactis and pMG36e-TSOL18/Lactococcus lactis Vaccines of Taenia solium.J Immunol Res. 2018 Nov 18;2018:9262631. doi: 10.1155/2018/9262631. eCollection 2018. J Immunol Res. 2018. PMID: 30581878 Free PMC article.
References
-
- Afonso, L. C., T. M. Scharton, L. Q. Vieira, M. Wysocka, G. Trinchieri, and P. Scott. 1994. The adjuvant effect of interleukin-12 in a vaccine against Leishmania major. Science 263:235-237. - PubMed
-
- al-Saleh, W., S. L. Giannini, N. Jacobs, M. Moutschen, J. Doyen, J. Boniver, and P. Delvenne. 1998. Correlation of T-helper secretory differentiation and types of antigen-presenting cells in squamous intraepithelial lesions of the uterine cervix. J. Pathol. 184:283-290. - PubMed
-
- Arulanandam, B. P., M. O'Toole, and D. W. Metzger. 1999. Intranasal interleukin-12 is a powerful adjuvant for protective mucosal immunity. J. Infect. Dis. 180:940-994. - PubMed
-
- Barth, H., R. Klein, P. A. Berg, B. Wiedenmann, U. Hopf, and T. Berg. 2001. Induction of T helper cell type 1 response and elimination of HBeAg during treatment with IL-12 in a patient with therapy-refractory chronic hepatitis B. Hepatogastroenterology 48:553-555. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

