The Apa protein of Mycobacterium tuberculosis stimulates gamma interferon-secreting CD4+ and CD8+ T cells from purified protein derivative-positive individuals and affords protection in a guinea pig model
- PMID: 12654810
- PMCID: PMC152084
- DOI: 10.1128/IAI.71.4.1929-1937.2003
The Apa protein of Mycobacterium tuberculosis stimulates gamma interferon-secreting CD4+ and CD8+ T cells from purified protein derivative-positive individuals and affords protection in a guinea pig model
Abstract
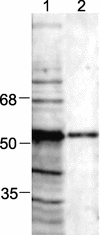
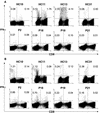
The search to identify Mycobacterium tuberculosis antigens capable of conferring protective immunity against tuberculosis has received a boost owing to the resurgence of tuberculosis over the past two decades. It has long been recognized that lymphoid cells are required for protection against M. tuberculosis. While traditionally the CD4(+) populations of T cells were believed to predominantly serve this protective function, a pivotal role for CD8(+) T cells in this task has been increasingly appreciated. We show that the 50- to 55-kDa Apa protein, specified by the Rv1860 gene of M. tuberculosis, can elicit both lymphoproliferative response and gamma interferon (IFN-gamma) production from peripheral blood mononuclear cells (PBMC) of purified protein derivative (PPD)-positive individuals, with significant differences recorded in the levels of responsiveness between PPD-positive healthy controls and pulmonary tuberculosis patients. Flow cytometric analysis of whole blood stimulated with the recombinant Apa protein revealed a sizeable proportion of CD8(+) T cells in addition to CD4(+) T cells contributing to IFN-gamma secretion. PBMC responding to the Apa protein produced no interleukin-4, revealing a Th1 phenotype. A DNA vaccine and a poxvirus recombinant expressing the Apa protein were constructed and tested for their ability to protect immunized guinea pigs against a challenge dose of virulent M. tuberculosis. Although the DNA vaccine afforded little protection, the poxvirus recombinant boost after DNA vaccine priming conferred a significant level of protective immunity, bringing about a considerable reduction in mycobacterial counts from the challenge bacilli in spleens of immunized guinea pigs, a result comparable to that achieved by BCG vaccination.
Figures


Similar articles
-
Protection against Mycobacterium tuberculosis infection offered by a new multistage subunit vaccine correlates with increased number of IFN-γ+ IL-2+ CD4+ and IFN-γ+ CD8+ T cells.PLoS One. 2015 Mar 30;10(3):e0122560. doi: 10.1371/journal.pone.0122560. eCollection 2015. PLoS One. 2015. PMID: 25822536 Free PMC article.
-
Aerosol vaccination with AERAS-402 elicits robust cellular immune responses in the lungs of rhesus macaques but fails to protect against high-dose Mycobacterium tuberculosis challenge.J Immunol. 2014 Aug 15;193(4):1799-811. doi: 10.4049/jimmunol.1400676. Epub 2014 Jul 14. J Immunol. 2014. PMID: 25024382 Free PMC article.
-
The Secreted Protein Rv1860 of Mycobacterium tuberculosis Stimulates Human Polyfunctional CD8+ T Cells.Clin Vaccine Immunol. 2016 Apr 4;23(4):282-93. doi: 10.1128/CVI.00554-15. Print 2016 Apr. Clin Vaccine Immunol. 2016. PMID: 26843486 Free PMC article.
-
[Novel vaccines against M. tuberculosis].Kekkaku. 2006 Dec;81(12):745-51. Kekkaku. 2006. PMID: 17240920 Review. Japanese.
-
Antigen-specific CD8(+) T cells and protective immunity to tuberculosis.Adv Exp Med Biol. 2013;783:141-63. doi: 10.1007/978-1-4614-6111-1_8. Adv Exp Med Biol. 2013. PMID: 23468108 Free PMC article. Review.
Cited by
-
Deciphering the role of VapBC13 and VapBC26 toxin antitoxin systems in the pathophysiology of Mycobacterium tuberculosis.Commun Biol. 2024 Oct 30;7(1):1417. doi: 10.1038/s42003-024-06998-6. Commun Biol. 2024. PMID: 39478197 Free PMC article.
-
Mycobacterium tuberculosis conserved hypothetical protein rRv2626c modulates macrophage effector functions.Immunology. 2010 May;130(1):34-45. doi: 10.1111/j.1365-2567.2009.03196.x. Epub 2010 Feb 26. Immunology. 2010. PMID: 20201990 Free PMC article.
-
Cellular immune responses to nine Mycobacterium tuberculosis vaccine candidates following intranasal vaccination.PLoS One. 2011;6(7):e22718. doi: 10.1371/journal.pone.0022718. Epub 2011 Jul 25. PLoS One. 2011. PMID: 21799939 Free PMC article.
-
Biosafety and Proteome Profiles of Different Heat Inactivation Methods for Mycobacterium tuberculosis.Microbiol Spectr. 2021 Dec 22;9(3):e0071621. doi: 10.1128/spectrum.00716-21. Epub 2021 Dec 22. Microbiol Spectr. 2021. PMID: 34937194 Free PMC article.
-
Surface proteome of "Mycobacterium avium subsp. hominissuis" during the early stages of macrophage infection.Infect Immun. 2012 May;80(5):1868-80. doi: 10.1128/IAI.06151-11. Epub 2012 Mar 5. Infect Immun. 2012. PMID: 22392927 Free PMC article.
References
-
- Altare, F., E. Jouanguy, S. Lamhamedi, R. Doffinger, A. Fischer, and J. L. Casanova. 1998. Mendelian susceptibility to mycobacterial infection in man. Curr. Opin. Immunol. 10:413-417. - PubMed
-
- Amara, R. R., S. Shanti, and V. Satchidanandam. 1998. Characterization of novel immunodominant antigens of Mycobacterium tuberculosis. Microbiol. 144(Pt. 5):1197-1203. - PubMed
-
- Bloom, B. R., and B. Bennett. 1966. Mechanism of a reaction in vitro associated with delayed-type hypersensitivity. Science 153:80-82. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

