The stress-responsive dgk gene from Streptococcus mutans encodes a putative undecaprenol kinase activity
- PMID: 12654811
- PMCID: PMC152025
- DOI: 10.1128/IAI.71.4.1938-1943.2003
The stress-responsive dgk gene from Streptococcus mutans encodes a putative undecaprenol kinase activity
Abstract
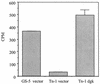
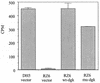
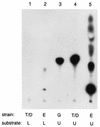
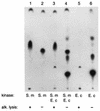
We analyzed a previously constructed stress-sensitive Streptococcus mutans mutant Tn-1 strain resulting from disruption by transposon Tn916 of a gene encoding a protein exhibiting amino acid sequence similarity to the Escherichia coli diacylglycerol kinase. It was confirmed that the mutation led to significantly reduced lipid kinase activity, while expression of the intact gene on a plasmid restored both kinase activity and the wild-type phenotype. Further analysis revealed that the product of the dgk gene in S. mutans predominantly recognizes a lipid substrate other than diacylglycerol, most likely undecaprenol, as demonstrated by its efficient phosphorylation and the resistance of the product of the reaction to saponification. The physiological role of the product of the dgk gene as a putative undecaprenol kinase was further supported by a significantly higher sensitivity of the mutant to bacitracin compared with that of the parental strain.
Figures

References
-
- Amann, E., B. Ochs, and K. J. Abel. 1988. Tightly regulated tac promoter vectors useful for the expression of unfused and fused proteins in Escherichia coli. Gene 69:301-315. - PubMed
-
- Badola, P., and C. R. Sanders II. 1997. Escherichia coli diacylglycerol kinase is an evolutionarily optimized membrane enzyme and catalyzes direct phosphoryl transfer. J. Biol. Chem. 272:24176-24182. - PubMed
-
- Bligh, E. G., and W. J. Dyer. 1959. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37:911-917. - PubMed
-
- Bohnenberger, E., and H. Sandermann, Jr. 1979. Diglyceride kinase from Escherichia coli. Purification in organic solvent and some properties of the enzyme. Eur. J. Biochem. 94:401-407. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

