Signaling through the T1/ST2 molecule is not necessary for Th2 differentiation but is important for the regulation of type 1 responses in nonhealing Leishmania major infection
- PMID: 12654814
- PMCID: PMC152039
- DOI: 10.1128/IAI.71.4.1961-1971.2003
Signaling through the T1/ST2 molecule is not necessary for Th2 differentiation but is important for the regulation of type 1 responses in nonhealing Leishmania major infection
Abstract
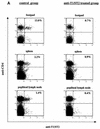
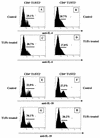
T1/ST2 is a stable cell surface marker selectively expressed on type 2 T helper (Th2) effector cells. Since nonhealing Leishmania major infections in susceptible BALB/c mice have been ascribed to a polarized Th2 response, we used an anti-T1/ST2 monoclonal antibody (MAb) or a T1-Fc fusion protein to investigate the role of CD4+ T1/ST2(+) Th2 cells in experimental leishmaniasis. We show that interfering with T1/ST2 signaling had no effect on lesion development or parasite replication; however, it induced a significantly higher type 1 response and an enhanced capacity of CD4+ T cells to respond to interleukin 12 (IL-12). Surprisingly, even in the presence of an elevated Th1 response, the production of antigen-specific type 2 cytokines was not altered in the group of mice treated with the anti-T1/ST2 MAb or the T1-Fc fusion protein. To characterize further this Th2 response, we assessed the cytokine profile of CD4+ T cells and found that interfering with T1/ST2 signaling did not alter the cytokine profile of CD4+ T1/ST2(+) T cells. These results show that T1/ST2 signaling is not necessary for the differentiation of naive CD4+ T cells into antigen-specific CD4+ T1/ST2(+) Th2 cells. In addition to CD4+ T1/ST2(+) T cells, we detected another subpopulation of CD4+ Th2 cells, negative for the expression of T1/ST2, that could differentiate in vivo in response to L. major infection. Taken together, our results suggest that CD4+ T1/ST2(+) Th2 cells but not CD4+ T1/ST2(-) Th2 cells can downregulate the Th1 response during the course of a nonhealing L. major infection through a mechanism that is independent of IL-4 or IL-10.
Figures




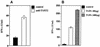
References
-
- Coyle, A. J., C. Lloyd, T. Nguyen, C. Erikson, L. Wang, P. Ottonson, P. Persson, T. Delaney, S. Lehar, S. Lin, L. Poisson, C. Meisel, T. Kamradt, T. Bjerke, D. Levinson, and J.-C. Gutierrez-Ramos. 1999. Crucial role of IL-1 receptor family member T1/ST2 in T helper cell type 2-mediated lung mucosal immune response. J. Exp. Med. 190:895-902. - PMC - PubMed
-
- Etges, B., and I. Müller. 1998. Progressive disease or protective immunity to Leishmania major infection: the result of a network of stimulatory and inhibitory interactions. J. Mol. Med. 76:372-390. - PubMed
-
- Himmelrich, H., P. Launois, I. Maillard, T. Biedermann, F. Tacchini-Cottier, R. M. Locksley, M. Röcken, and J. A. Louis. 2000. In BALB/c mice, IL-4 production during the initial phase of infection with L. major is necessary and sufficient to instruct Th2 cell development resulting in progressive disease. J. Immunol. 164:4819-4825. - PubMed
-
- Hondowicz, B., A. Y. Park, M. M. Elloso, and P. Scott. 2000. Maintenance of IL-12-responsive CD4+ T cells during a Th2 response in L. major-infected mice. Eur. J. Immunol. 30:2007-2014. - PubMed
-
- Hondowicz, B. D., T. M. Scharton-Kersten, D. Jones, and P. Scott. 1997. Leishmania major-infected C3H mice treated with anti-IL-12 mAb develop but do not maintain a Th2 response. J. Immunol. 159:5024-5031. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

